Advertisements
Advertisements
प्रश्न
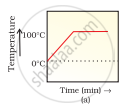
A student heats a beaker containing ice and water. He measures the temperature of the content of the beaker as a function of time. Which of the following (Fig. 1.1) would correctly represent the result? Justify your choice.
पर्याय
उत्तर
Since ice and water are in equilibrium, the temperature would be zero. When we heat the mixture, ‘energy supplied is utilised in melting the ice and the temperature does not change till the ice melts because of latent heat of fusion. On further heating the temperature of the water would increase. Therefore (d) is the correct option.
APPEARS IN
संबंधित प्रश्न
The temperature at which a solid convert into a liquid is called its .........
Molecules of a substance are always in a state of............ and so they possess .............
Differentiate between melting point ,giving atleast one example of each.
Explain the term Melting.
Conversion of solid state to liquid state is called fusion; what is meant by latent heat of fusion?
In an endothermic process, the speed of the molecules is ______ hence they move faster.
A puddle of water gets pooled around the glass of ice cream or a glass of ice cubes. When it is kept at room temperature. Give reason.
The solid, liquid, and phases of water can coexist in equilibrium at ______.
Define latent heat of fusion?
Explain the following effects of heat.
Change in state