Advertisements
Advertisements
Question
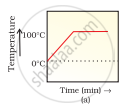
A student heats a beaker containing ice and water. He measures the temperature of the content of the beaker as a function of time. Which of the following (Fig. 1.1) would correctly represent the result? Justify your choice.
Options
Solution
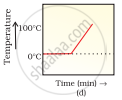
Since ice and water are in equilibrium, the temperature would be zero. When we heat the mixture, ‘energy supplied is utilised in melting the ice and the temperature does not change till the ice melts because of latent heat of fusion. On further heating the temperature of the water would increase. Therefore (d) is the correct option.
APPEARS IN
RELATED QUESTIONS
Molecules of a substance are always in a state of............ and so they possess .............
Differentiate between melting point ,giving atleast one example of each.
Some ice is taken in a beaker and its temperature is recorded after each one minute. The observations are listed below
| Time (in minute) | Temperature (in °C) |
| 0 | 0 |
| 1 | 0 |
| 2 | 0 |
| 3 | 0 |
| 4 | 0 |
| 5 | 0 |
| 6 | 3.8 |
| 7 | 7.6 |
| 8 | 11.4 |
From the above observations, what conclusion do you draw about the melting point of ice?
State the melting point of ice.
What is melting? Give example.
A glass tumbler containing hot water is kept in the freezer compartment of a refrigerator (temperature < 0°C). If you could measure the temperature of the content of the tumbler, which of the following graphs (Fig.1.2) would correctly represent the change in its temperature as a function of time.
In an endothermic process, the speed of the molecules is ______ hence they move faster.
Sweating causes cooling because water has a ______.
A change of state is a change of a substance from ______.
Define latent heat of fusion?