Advertisements
Advertisements
प्रश्न
A solid of mass 50 g at 150 °C is placed in 100 g of water at 11 °C when the final temperature recorded is 20 °C. Find the specific heat capacity of the solid. (specific heat capacity of water = 4.2 J/g °C)
उत्तर
Mass of the solid, ms = 50 g
Initial temperature of the solid, ts = 150C
Mass of water, mw = 100 g
Temperature of water, tw = 11°C
Final temperature of the mixture, t = 20°C
According to the principle of calorimetry
Heat gained by water = Heat lost by the solid
∴ 100 x 4.2 x (20 - 10) = 50 x cs x (150 - 20)
3780 = 6500cs
APPEARS IN
संबंधित प्रश्न
Explain the term boiling point ?
Ice cream appears colder to the mouth than water at 0℃. Give reason.
The product of mass and specific heat is known as ..........
A substance is heated at a constant rate from a low temperature to a high temperature. A graph of temperature against time is shown in the figure. Which part or parts of the graph correspond(s) to the substance existing in two states?
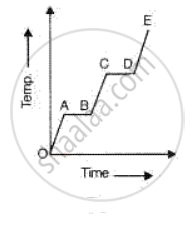
Explain, why water is considered as best liquid for quenching thirst?
Solve the following problem.
What is the specific heat of metal if 50 cal of heat is needed to raise 6 kg of the metal from 20°C to 62 °C?
A monoatomic gas of pressure 'P' having volume 'V' expands isothermally to a volume '2V' and then adiabatically to a volume '16V'. The final pressure of the gas is ______.
(ratio of specific heats =
The specific heat capacity of ______ is maximum.
