Advertisements
Advertisements
प्रश्न
Acetic acid is a typical acid. Write one equation in case of its reactions with a carbonate.
उत्तर
\[\ce{2CH3COOH + \underset{Sodium carbonate}{Na2CO3} -> \underset{Sodium acetate}{2CH3COONa} + H2O + CO2}\]
APPEARS IN
संबंधित प्रश्न
For preparing soap in the laboratory we require an oil and a base. Which of the following combinations of an oil and a base would be best suited for the preparation of soap?
(a) Castor oil and calcium hydroxide
(b) Turpentine oil and sodium hydroxide
(c) Castor oil and sodium hydroxide
(d) Mustard oil and calcium hydroxide
A student adds 2 mL of acetic acid to a test tube containing 2 mL of distilled water. He then shakes the test tube well and leaves it to settle for some time. After about 5 minutes he observes that in the test tube there is :
(A) a clear transparent colourless solution
(B) a clear transparent pink solution
(C) a precipitate settling at the bottom of the test tube
(D) a layer of water the layer of acetic acid
Complete the following chemical equations: CH3COOH+NaHCO3→
Name the gas evolved when ethanoic acid is added to sodium carbonate. How would you prove the presence of this gas?
Complete the following equation:
`CH_3 COOH + CH_2 H_5 OH` 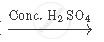
Name the reducing agent used to convert acetic acid into ethanol?
Name the substance used to change acetic acid to acetic anhydride?
Choose the correct alternative and rewrite the following:
Some acetic acid is treated with solid NaHCO3, the resulting solution will be _________________.
Give the balanced chemical equation of the following reaction:
Neutralization of NaOH with ethanoic acid.
Mineral acids are stronger acids than carboxylic acids because
- mineral acids are completely ionised
- carboxylic acids are completely ionised
- mineral acids are partially ionised
- carboxylic acids are partially ionised
