Advertisements
Advertisements
प्रश्न
Answer the following in brief.
What is a salt bridge?
उत्तर
A salt bridge is a U tube containing a saturated solution of an inert electrolyte such as KCl or NH4NO3 , Na2SO4 in a solidified agar-agar gel. A hot saturated solution of these electrolytes in 5% agar solution is filled in the U-shaped tube and allowed it to cool and solidify forming a gel.
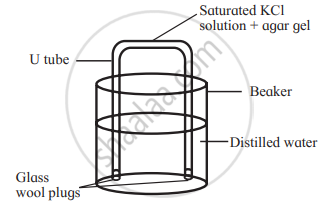
It is used to connect two half-cells or electrodes forming a galvanic or voltaic cell.
APPEARS IN
संबंधित प्रश्न
Answer the following in one or two sentences.
Write any two functions of salt bridge.
Answer the following in one or two sentences.
Formulate a cell from the following electrode reactions:
`"Au"_(("aq"))^(3+) + 3"e"^(-) -> "Au"_(("s"))`
Mg(s) → `"Mg"_(("aq"))^(2+)` + 2e-
Answer the following in one or two sentences.
What is the significance of the single vertical line and double vertical line in the formulation galvanic cell?
Calculate `"E"_"cell"^0` for galvanic cell with electrodes Co/Co3+// Mn2+/Mn.
`"E"_"Mn"^0` = - 1.18 V, `"E"_"Co"^0` = 1.82 V.
Give the chemical composition present in the salt bridge.
What are the functions of a salt bridge in a galvanic cell?
Represent the galvanic cell from following overall cell reaction:
3Ni(s) + 2Al3+ (1 M) → 3Ni2+(1 M) + 2Al(s)
Calculate standard cell potential of following galvanic cell:
Zn/Zn2+(1 M) // Pb2+(1 M)/Pb. If `"E"_"Pb"^0` = 0.126 V and `"E"_"Zn"^0` = –0.763 V
A single vertical line is used to denote __________ in the cell notation of galvanic cell.
In the reaction, \[\ce{Cu_{(s)} + 2Ag^+_{( aq)} -> Cu^{2+}_{( aq)} + 2Ag_{(s)}}\]; the reduction half-cell reaction:
Select the INCORRECT statement regarding galvanic cell notation.
The \[\ce{E^0_{cell}}\] for the following cell is:
\[\ce{Fe_{(s)} | Fe^{2+}_{( aq)} || Zn^{2+}_{( aq)} | Zn_{(s)}}\]
\[\ce{E^0_{Fe}}\] = −0.41 V,
\[\ce{E^0_{Zn}}\] = −0.76 V
Which of the following scientic notation of some figures is not correct?
Formulate the galvanic cell in which the following reaction occurs.
2Al(S) + 3Cu2+(aq) → 2Al3+(aq) + 3Cu(S)
A voltaic cell consisting of Fe2+(aq)| Fe(s) and Bi3+(aq)| Bi(s) electrodes is constructed. When the circuit is closed, mass of Fe electrode decreases and that of Bi electrode increases.
- Write cell formula .
- Which electrode is cathode and which electrode is anode?
- Write electrode reactions and overall cell reaction.
Write two uses of salt bridge.
