Advertisements
Advertisements
प्रश्न
Arrange the following in decreasing order of their acidic strength and give reason for your answer.
उत्तर
The order is
When compared to other alcohols
APPEARS IN
संबंधित प्रश्न
Name the following compound according to IUPAC system of nomenclature:
(CH3)3CCH2COOH
Benzophenone can be obtained by:
(i) Benzoyl chloride + Benzene +
(ii) Benzoyl chloride + Diphenyl cadmium
(iii) Benzoyl chloride + Phenyl magnesium chloride
(iv) Benzene + Carbon monoxide +
Arrange the following in the increasing order of their property indicated:
Acetaldehyde, Acetone, Methyl tert butyl ketone (reactivity towards NH2OH).
The correct IUPAC name for Na[PdBrCl(NO2)(NH3)] is:
IUPAC name of following compound is: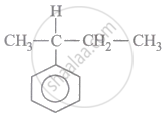
Write the reaction and IUPAC name of the product formed when 2-Methylpropanal (isobutyraldehyde) is treated with ethyl magnesium bromide followed by hydrolysis.
Write the structure of the following compound.
Di-sec. butyl ketone
Draw structure of the following derivative.
Acetaldehydedimethylacetal.
Write the structure of the following compound.
Di-sec. butyl ketone
Write the structure of the following compound:
Di-sec. butyl ketone
