Advertisements
Advertisements
प्रश्न
Arrange the following in decreasing order of their boiling points.
(A) n-butane
(B) 2-methylbutane
(C) n-pentane
(D) 2, 2-dimethylpropane
पर्याय
A > B > C > D
B > C > D > A
D > C > B > A
C > B > D > A
उत्तर
C > B > D > A
Explanation:
As the number of carbon atom increases, boiling point increases. Boiling point decreases with branching.
2, 2-dimethyl propane
\[\begin{array}{cc}
\phantom{...}\ce{CH3}\\
\phantom{}|\\
\ce{H3C - C - CH3}\\
\phantom{}|\\
\phantom{...}\ce{\underset{b.pt = 282.5 K}{CH3}}
\end{array}\]
n-pentane
\[\ce{\underset{b.pt = 309.1 K}{H3C - CH2 - CH2 - CH2 - CH3}}\]
2-methyl butane
\[\begin{array}{cc}
\ce{H3C - H2C - HC - CH3}\\
\phantom{.........}|\\
\phantom{............}\ce{\underset{b.pt = 301 K}{CH3}}
\end{array}\]
n-butane
\[\ce{\underset{(4 carbon atoms with no branching)}{\underset{b.pt = 273 K}{H3C - H2C - CH2 - CH3}}}\]
APPEARS IN
संबंधित प्रश्न
Write IUPAC name of the following compound:
CH2 = CH - C ≡ C - CH3
Write IUPAC name of the following compound:

Write IUPAC name of the following compound:
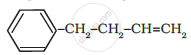
Write IUPAC name of the following compound:

Write IUPAC name of the following compound:
\[\begin{array}{cc}
\ce{CH3(CH2)4 CH(CH2)3 CH3}\\
|\phantom{..}\\
\phantom{..............}\ce{CH2 - CH(CH3)2}
\end{array}\]
Write IUPAC name of the following compound:
\[\begin{array}{cc}
\ce{CH3 - CH = CH - CH2 - CH = CH - CH - CH2 - CH = CH2}\\
\phantom{.................}|\\\phantom{.....................}\ce{C2H5}\end{array}\]
Match the reagent from Column I which on reaction with CH3 – CH = CH2 gives some product given in Column II as per the codes given below:
| Column I | Column II |
| (i) O3/Zn + H2O | (a) Acetic acid and CO2 |
| (ii) KMnO4/H+ | (b) Propan-1-ol |
| (iii) KMnO4/OH– | (c) Propan-2-ol |
| (iv) H2O/H+ | (d) Acetaldehyde and formaldehyde |
| (v) B2H6/NaOH and H2O2 | (e) Propane-1, 2-diol |
Assertion (A): Among isomeric pentanes, 2, 2-dimethylpentane has highest boiling point.
Reason (R): Branching does not affect the boiling point.
Observed structures of the following compounds:
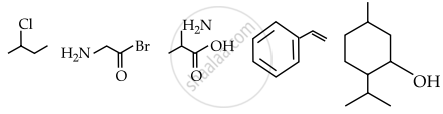
The total number of structures/compounds which possess asymmetric carbon atoms.
An alkene ‘A’ contains three C – C, eight C – H σ bonds and one C – C π bond. ‘A’ on ozonolysis gives two moles of an aldehyde of molar mass 44 u. Write IUPAC name of ‘A’.
