Advertisements
Advertisements
प्रश्न
Arrange the halogens F2, Cl2, Br2, I2, in order of their increasing reactivity with alkanes.
पर्याय
I2 < Br2 < Cl2 < F2
Br2 < Cl2 < F2 < I2
F2 < Cl2 < Br2 < I2
Br2 < I2 < Cl2 < F2
उत्तर
I2 < Br2 < Cl2 < F2
Explanation:
The reactivity of halogens with alkanes depends on the electronegativity i.e. higher the electronegativity higher would be the reaction. As down the group reactivity decrease so thus its reactivity.
APPEARS IN
संबंधित प्रश्न
Write IUPAC name of the product obtained by the ozonolysis of the following compound.
Pent-2-ene
Propanal and pentan-3-one are the ozonolysis products of an alkene? What is the structural formula of the alkene?
Write a chemical equation for combustion reaction of the following hydrocarbon:
Butane
Which of the following alkenes on ozonolysis give a mixture of ketones only?
| (i) | CH3 – CH = CH – CH3 |
| (ii) | \[\begin{array}{cc} \ce{CH3 - C - CH = CH2}\\ |\phantom{.......}\\ \ce{CH3}\phantom{....} \end{array}\] |
| (iii) | 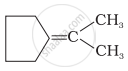 |
| (iv) | \[\begin{array}{cc} \phantom{...................}\ce{CH3}\\ \phantom{..............}/\\ \ce{(CH3)2 C = C}\\ \phantom{..............}\backslash\\ \phantom{...................}\ce{CH3} \end{array}\] |
Which of the following reagent is used for the following reaction?
\[\ce{CH3CH2CH3 ->[?] CH3CH2CHO}\]
The major product formed in the following reactions is:
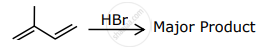
What would be the main product when propene reacts with HBr?
3-Methyl-pent-2-ene on reaction with HBr in presence of peroxide forms an addition product. The number of possible stereoisomers for the product is ______.
An alkene ‘A’ contains three C – C, eight C – H σ bonds and one C – C π bond. ‘A’ on ozonolysis gives two moles of an aldehyde of molar mass 44 u. Write the IUPAC name of ‘A’.
An alkene ‘A’ contains three C-C, eight C-H σ bonds and one C-C π bond. ‘A’ on ozonolysis gives two moles of an aldehyde of molar mass 44 u. Write the IUPAC name of ‘A’.
