Advertisements
Advertisements
प्रश्न
Assertion: Chlorobenzene is resistant to nucleophilic substitution reaction at room temperature.
Reason (R): C–Cl bond gets weaker due, to resonance.
पर्याय
Both (A) and (R) are true and (R) is the correct explanation of (A).
Both (A) and (R) are true, but (R) is not the correct explanation of (A).
(A) is true, but (R) is false.
(A) is false, but (R) is true.
उत्तर
(A) is true, but (R) is false.
Explanation:
Because of resonance, chlorobenzene acquires a partial double bond, making it extremely reactive to electrophilic substitution reactions.
APPEARS IN
संबंधित प्रश्न
Write the final product(s) in each of the following reactions:
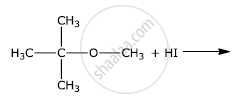
The presence of nitro group (−NO2) at o/p positions increases the reactivity of haloarenes towards nucleophilic substitution reactions.
How the following conversion can be carried out?
Chlorobenzene to p-nitrophenol
Give reasons:
The dipole moment of chlorobenzene is lower than that of cyclohexyl chloride.
Out of (CH3)3 C-Br and (CH3)3 C-I, which one is more reactive towards SN1 and why?
Which of the following compounds will give racemic mixture on nucleophilic substitution by \[\ce{OH-}\] ion?
(a) \[\begin{array}{cc}
\phantom{}\ce{CH3 - CH - Br}\\
\phantom{}|\\
\phantom{....}\ce{C2H5}\phantom{}
\end{array}\]
(b) \[\begin{array}{cc}
\phantom{..}\ce{Br}\\
\phantom{}|\\
\phantom{}\ce{CH3 - C - CH3}\\
\phantom{}|\\
\phantom{....}\ce{C2H5}\phantom{}
\end{array}\]
(c) \[\begin{array}{cc}
\phantom{....}\ce{CH3 - CH - CH2Br}\\
\phantom{}|\\
\phantom{....}\ce{C2H5}\phantom{}
\end{array}\]
Arrange the following compounds in increasing order of rate of reaction towards nucleophilic substitution.
| (a) | 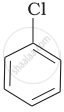 |
| (b) | 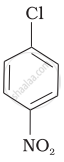 |
| (c) | 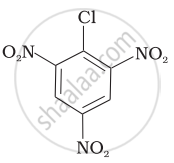 |
Arrange the following compounds in increasing order of rate of reaction towards nucleophilic substitution.
| (a) | 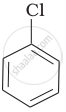 |
| (b) | 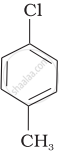 |
| (c) | 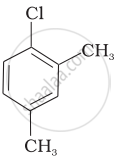 |
Haloarenes are less reactive than haloalkanes and haloalkenes. Explain.
Why haloarenes are not reactive towards nucleophilic substitution reaction? Give two reactions.
