Advertisements
Advertisements
प्रश्न
Choose the correct option from the bracket and explain the statement giving a reason.
(Oxidation, displacement, electrolysis, reduction, zinc, copper, double displacement, decomposition)
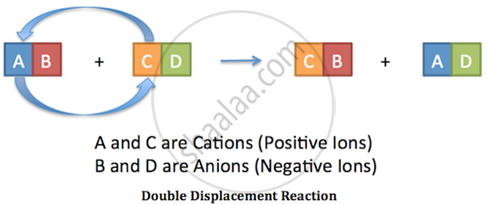
Addition of an aqueous solution of ZnSO4 to an aqueous solution of BaCl2 is an example of ______ reaction.
उत्तर
Addition of an aqueous solution of ZnSO4 to an aqueous solution of BaCl2 is an example of double displacement reaction.
Explanation:
On addition of ZnSO4 and BaCl2, the following reaction takes place:
In the reaction, the exchange of ions is taking place. The reaction in which the exchange of ions takes place to form precipitate are called double displacement reaction.
संबंधित प्रश्न
Complete and balance the following chemical equations:
Zn + `"H"_2"SO"_4` `→`
(dil)
FILL IN THE BLANK
The new substances formed during a chemical reaction are called the ..................
FILL IN THE BLANK
Distillation is a method of obtaining pure ............. from a solution.
State four conditions necessary for chemical reactions to take place.
Write true or false :
No new substance is formed during a chemical reaction.
A solution of a substance ‘X’ is used for white washing write the reaction of the substance ‘X’ named in (a) above with water.
Show with the help of diagram of electronic configuration how the following compound is formed from the constituent atoms.
Water
Choose the correct option from the bracket and explain the statement giving reason.
(Oxidation, displacement, electrolysis, reduction, zinc, copper, double displacement, decomposition)
The conversion of ferrous sulphate to ferric sulphate is ______ reaction.
Give an example of a reaction where the following are involved
Light
Define Photochemical reaction
Give an example
Give an example of the following chemical change.
A reaction where colour change is noticed
Complete and balance the following reaction:
\[\ce{Pb(NO3)2 ->[\Delta]}\]
What is observed in performing the following :
| Hydrogen | Oxygen | Carbon dioxide | Chlorine | |
| Litmus test | ||||
| Apply a burning splint to the gas | ||||
| Colour of gas | colourless | colourless | colourless | greenish-yellow |
| Odour of gas |
State the following reaction represent oxidation or reduction.
\[\ce{Cl- ->Cl}\]
Select the correct answer from the options given below:
State the gaseous product formed, when
An active metal reacts with dilute sulphuric acid.
Anaerobic bacteria digest animal waste and produce biogas (Change – A). The biogas is then burnt as fuel (Change – B). The following statements pertain to these changes. Choose the correct one.
Water boiling into water vapour is an example of chemical change.
Give two examples for each of the following cases:
Chemical changes.
The simplest method of preventing rusting of iron is to coat it with oil, grease, or paint. The reason being ______.
Iron and rust are the same substances.
