Advertisements
Advertisements
Question
Choose the correct option from the bracket and explain the statement giving a reason.
(Oxidation, displacement, electrolysis, reduction, zinc, copper, double displacement, decomposition)
Addition of an aqueous solution of ZnSO4 to an aqueous solution of BaCl2 is an example of ______ reaction.
Solution
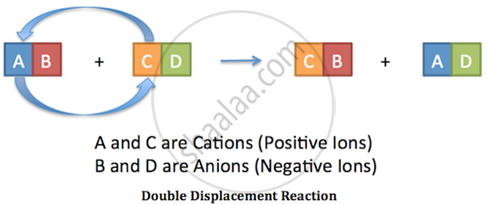
Addition of an aqueous solution of ZnSO4 to an aqueous solution of BaCl2 is an example of double displacement reaction.
Explanation:
On addition of ZnSO4 and BaCl2, the following reaction takes place:
In the reaction, the exchange of ions is taking place. The reaction in which the exchange of ions takes place to form precipitate are called double displacement reaction.
RELATED QUESTIONS
Complete and balance the following chemical equations:
N2 + O2 →
FILL IN THE BLANK
The new substances formed during a chemical reaction are called the ..................
MATCH THE COLUMNS
| 1 . `H_2` + `CI_2` `→` 2HCI | a. displacement reaction |
| 2. `2FeCI_3`+ `H_2` `→` `2FeCI_2` +2HCI` | b. addition of an electronegative redical |
| 3. Fe + `CuSO_4` `→``FeSO_4` +Cu` | c. removal of an electronegative redical |
| 4. `2H_2O` +`4NO_2`+`O_2` `→` `4HNO_3` | d.reduction by adding hydrogen |
| 5. 3Fe +`3CI_2` `→` `2FeCI_3` | e.electrolysis of water |
| 6. `FeCI_3`+ 3NaOH `→``Fe(OH)_3``↓`+NaCI | F.double decomposition,precipitation reaction |
| 7.`2H_2O` `→` `2H_2``↑`+`O_2` | e. combination or synthesis reaction |
Complete the statement by filling the gaps using appropriate term from the terms given in the bracket.
(slow, coloured, arrow, fast, smell, milky, physical, product, chemical, reactant, covalent, ionic, octet, duplet, exchange, sharing, equality sign)
Chlorine (Cl2) molecule is formed by ______ of electrons between two chlorine atoms.
Explain by writing a word equation.
Bubbles are seen on adding lemon juice to baking soda.
Show with the help of diagram of electronic configuration how the following compound is formed from the constituent atoms.
Water
Multiple choice:
Which of the following is not a characteristic of a chemical change?
Complete and balance the following reaction:
\[\ce{NH3 + O2 ->[\Delta]}\]
Name: A greenish-yellow gas.
What do you observe when Cl2 is passed through potassium iodide (KI) solution.
Distinguish by heating the following in dry test tubs.
Copper Sulphate and copper carbonate
A chemical reaction is often accompanied by external indications or characteristics which include –
- Colour change
- Effervescence or gas evolved
- Evolution or absorption of heat
- Formation of a precipitate.
With reference to each of the above indications, state the external indication seen during – Thermal decomposition of mercury [II] oxide.
A chemical reaction is often accompanied by external indications or characteristics.
Silver metal can displace hydrogen gas from nitric acid.
The changes in which new substance with new chemical properties are formed are ______.
Match the following:
| 1. | Sodium Chloride | a. | CaSO41/2H2O |
| 2. | Calcium sulphate dihydrate | b. | C2H5Oh |
| 3. | Magnesium sulphate hydrate | c. | CaSO4.2H2O |
| 4. | Calcium sulphate hemihydrate | d. | NaCl |
| 5. | Phenol | e. | MgSO4.H2O |
Give an example where heat, light, and sound are produced during a chemical change.
When carbon dioxide is passed through lime water ______.
______ is the chemical name of rust.
When milk is mixed with coffee decoction, the colour of milk and decoction changes due to ______.
