Advertisements
Advertisements
प्रश्न
Draw the structural formula of a compound with two carbon atoms in the following case.
An unsaturated hydrocarbon with carbon to carbon triple bond
उत्तर
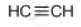
APPEARS IN
संबंधित प्रश्न
Give the structural formulae of 2-methylpropane.
Give balanced chemical equations for the ethanoic acid to ethyl ethanoate.
Complete and balance the following equation. State the condition wherever necessary.
C2H4 + Br2 → ______
Complete and balance the following equation. State the condition wherever necessary.
C2H2 + Br2 → ______
Complete and balance the following equation. State the condition wherever necessary.
\[\ce{C2H5OH ->[O][K2Cr2O7]}\] ______
Give at least one example in case to show the structure of isomers of single-bond compounds.
What will be the formula and structure of benzene?
Draw the structural formulae of the two isomers of Butane. Give the correct IUPAC name of each of the isomer.
Name the following:
Organic compounds with same molecular formula but different structural formula.
The structures of four hydrocarbons are shown below:
| \[\begin{array}{cc} \phantom{.....}\ce{CH3}\\ \phantom{...}|\\ \ce{H3C - C - H}\\ \phantom{...}|\\ \phantom{.....}\ce{CH3} \end{array}\] |
\[\begin{array}{cc} \ce{CH3}\phantom{.....}\\ |\phantom{.......}\\ \ce{C = CH2}\\ |\phantom{.......}\\ \ce{CH3}\phantom{....} \end{array}\] |
\[\begin{array}{cc} \ce{H}\phantom{.....}\\ |\phantom{.....}\\ \ce{H3C - C - C - CH2}\\ |\phantom{....}|\\ \ce{H\phantom{...}H} \end{array}\] |
\[\begin{array}{cc} \phantom{...}\ce{CH3}\\ |\\ \ce{H3C - C = CH2} \end{array}\] |
How many isomers of butene are there?
