Advertisements
Advertisements
प्रश्न
Explain the bond formation if BeCl2 and MgCl2.
उत्तर
Bond formation of BeCl2:
Be = 4;
Electronic configuration of Be atom is = 1s2 2s2

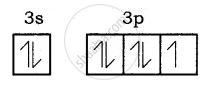

Bond formation of MgCl2:
Mg (Z = 12), 1s2 2s2 2p6 3s2 it loses two of its valence electron and became Mg2+ with inert gas configuration Neon.
The chlorine accepts one electron in its valence shell and because Cl– ion with Ar electron configuration.
Mg + Cl2 → Mg2+ + 2 Cl– → MgCl2
Magnesium cation and two chlorides are attracted by strong electrostatic force to form
MgCl2 crystals.
Mg = 12,
Electronic configuration: 1s2 2s2 2p6 3s2
Mg+2:
Electronic configuration: 1s2 2s2 2p6 3s0
Cl = 17, 1s2 2s2 2p6 3s2 3p5
Cl–:
Electronic configuration: 1s2 2s2 2p6 3s2 3p6


APPEARS IN
संबंधित प्रश्न
Which of the following molecule contain no л bond?
Draw the Lewis structure for the following species.
HNO3
Explain an example of a Polar Covalent bond.
Draw the Lewis structures for the following species.
\[\ce{NO^-3}\]
Draw the Lewis structure for the following species.
\[\ce{SO^2-_4}\]
Draw the Lewis structure for the following species.
\[\ce{NO^-3}\]
Draw the Lewis structure for the following species.
\[\ce{NO^-_3}\]
Draw the Lewis structure for the following species.
SO42-
Draw the Lewis structures for the following species:
\[\ce{SO^2-_4}\]
Draw the Lewis structure for the following species.
\[\ce{NO^-_3}\]
