Advertisements
Advertisements
प्रश्न
Explain the magnetic properties of [Ni(CN4)]2–.
उत्तर
Magnetic properties of [Ni(CN4)]2–:
- In [Ni(CN4)]2– ion, Ni2+ undergoes dsp2 hybridization.
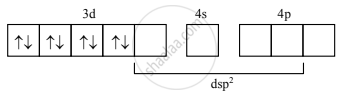
- Four vacant dsp2 hybrid orbitals of Ni2+ overlap with four orbitals of CN– ions to form Ni–CN coordinate bonds.
Configuration after the complex formation becomes:
- The complex has no unpaired electrons and hence, diamagnetic.
APPEARS IN
संबंधित प्रश्न
Choose the most correct option.
Which of the following complexes is chiral?
1. [Co(en)2Cl2]⊕
2. [Pt(en)Cl2]
3. [Cr(C2O4)3]3-
4. [Co(NH3)4Cl2]⊕
Answer the following in one or two sentences.
Is the complex [CoF6] cationic or anionic if the oxidation state of cobalt ion is +3?
Answer in brief.
Classify the following complexes as homoleptic and heteroleptic [Cu(NH3)4]SO4, [Cu(en)2(H2O)Cl]2⊕, [Fe(H2O)5 (NCS)]2⊕, tetraammine zinc (II) nitrate.
Answer the following question.
What are cationic, anionic, and neutral complexes? Give one example of each.
Explain homoleptic and heteroleptic complexes.
Which of the following is CORRECT for [Co(NH3)5Br]Br2?
Identify the tetradentate ligand from the following.
Which statement from following is true for a complex hexaminecobalt (III) chloride?
Which among the following complexes is NOT a heteroleptic complex?
Which among the following compounds is cationic complex?
Identify cationic complex from the following.
Which coordinate complex from the following has a complex ion with positive charge?
How many points of attachment are present in the ligand dimethyl glyoximato?
Explain cationic, anionic and neutral sphere complexes w ith example.
The correct order of ligands in the spectrochemical series is ______.
What is the number of donor atoms in dimethylglyoximato ligand?
Explain the classification of coordination complexes on the basis of charge on the complex.
