Advertisements
Advertisements
प्रश्न
Explain the term – molecules. Give three examples of atoms of the same element forming a molecule. State the atomicity of the same.
उत्तर
Atoms of the same element or different elements combine to form a molecule.
Atoms of the same element forming a molecule
1. Oxygen
2. Nitrogen
3. Hydrogen.

Atomicity is the number of atoms present in one molecule of the element.
| Element | Molecule | Atomicity |
| Hydrogen H | H2 | 2 |
| Nitrogen N | N2 | 2 |
| Oxygen O | O2 | 2 |
संबंधित प्रश्न
Give the name of the element present in the following compound:
Quick lime
Define: Atomic number
Define: Atomic weight :
What is the difference between the molecule of an element and the molecule of a compound ? Give one
example of each.
What are (i) ionic compounds, and (ii) molecular compounds ? Give two examples of each type of compounds.
Molecular compounds are usually formed by the combination between :
Calculate the mass of 3.011 × 1024 atoms of carbon.
Why are the symbols and formulae of substances important?
Give one example of a triatomic molecule
Give one example of a polyatomic molecule.
State the atomicity of the following:
Neon
State the atomicity of the following:
Oxygen
State the atomicity of the following:
Chlorine
State the atomicity of the following:
Sulphur
Classify the following molecules as the molecules of element or compound
| 1. |  |
|
| 2. |  |
|
| 3. | 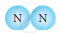 |
|
| 4. | 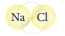 |
If a molecule is made of similar kinds of atoms, then it is called ______ atomic molecule.
Give any two examples for heterodiatomic molecules.
Write any two classifications of a molecule.
