Advertisements
Advertisements
प्रश्न
Express the change in internal energy in terms of molar specific heat capacity.
उत्तर
When the gas is heated at constant volume the temperature increases by dT. As no work is done by the gas, the heat that flows into the system will increase only the internal energy. Let the change in internal energy be dU. dU = µωdT
APPEARS IN
संबंधित प्रश्न
What amount of heat must be supplied to 2.0 x 10-2 kg of nitrogen (at room temperature) to raise its temperature by 45 °C at constant pressure? (Molecular mass of N2 = 28; R = 8.3 J mol-1 K-1.)
Calculate the mass of ice required to lower the temperature of 300 g of water 40°C to water at 0°C.
(Specific latent heat of ice = 336 J/g, the Specific heat capacity of water = 4.2J/g°C)
Explain the term boiling point ?
A burner raises the temperature of 360 g of water from 40°C to 100°C in 5 minutes. Calculate the rate of heat supplied by the burner.
Write the approximate values of the specific latent heat of fusion of ice.
Explain, why does a wise farmer water his fields, if forecast is forst?
63.2 g of copper at 50°C can just melt 3.8g of ice. If the specific latent heat of ice is 336 J/g, find the specific heat capacity of copper.
Water falls from a height of 50 m. Calculate the rise in the temperature of water when it strikes the bottom.
(g = 10 ms-2; Specific heat capacity of water = 4200 J / kg°C)
Decide the unit for specific heat capacity.
The diagram below shows a cooling curve for 200 g of water. The heat is extracted at the rate of 100 Js-1. Answer the questions that follow:
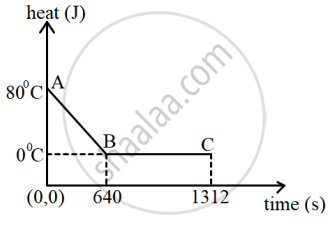
- Calculate specific heat capacity of water.
- Heat released in the region BC.
