Advertisements
Advertisements
Question
Express the change in internal energy in terms of molar specific heat capacity.
Solution
When the gas is heated at constant volume the temperature increases by dT. As no work is done by the gas, the heat that flows into the system will increase only the internal energy. Let the change in internal energy be dU. dU = µωdT
APPEARS IN
RELATED QUESTIONS
You have a choice of three metals A, B, and C, of specific heat capacities 900 Jkg-1 °C-1, 380 Jkg-1 °C-1 and 460 Jkg-1 °C-1 respectively, to make a calorimeter. Which material will you select? Justify your answer.
Name a liquid which has the highest specific heat capacity.
What impact will global warming have on the health of the affected population?
A liquid X has specific heat capacity higher than the liquid Y. Which liquid is useful as heat reservoir to keep juice bottles without freezing?
Some heat is provided to a body to raise its temperature by 25°C. What will be the corresponding rise in temperature of the body as shown on the Kelvin scale?
1 kg of water freezes to form ice at 0°C. What amount of heat is withdrawn?
A substance is in the form of a solid at 0°C. The amount of heat added to this substance and the temperature of the substance are plotted on the following graph:

If the specific heat capacity of the solid substance is 500 J/kg °G, find from the graph, the mass of the substance.
Read this activity and answer the following questions.
- Take three spheres of iron, copper and lead. the lead of equal mass.
- Put all the three spheres in boiling water in the beaker for some time.
- Take the three spheres out of the water.
- All the spheres will be at a temperature 100 °C.
- Put them immediately on the thick slab of wax.
- Note, the depth that each of the sphere goes into the wax.
Questions:
- Which property is determined from this activity?
- Give name to that property.
- Explain the term principal of heat exchange with the help of this activity.
Numerical Problem.
What is the heat in joules required to raise the temperature of 25 grams of water from 0°C to 100°C? What is the heat in Calories? (Specific heat of water = `(4.18"J")/("g"°"C")`
The diagram below shows a cooling curve for 200 g of water. The heat is extracted at the rate of 100 Js-1. Answer the questions that follow:
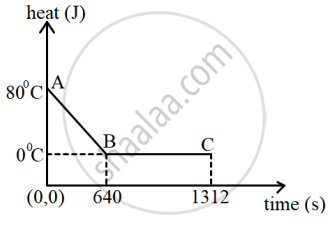
- Calculate specific heat capacity of water.
- Heat released in the region BC.
