Advertisements
Advertisements
प्रश्न
Give balanced chemical equations for the following conversions A, B, and C:
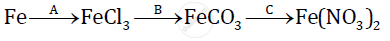
उत्तर
A:2Fe + 3Cl2 → 2FerCl3
B:2FeCl3 + 3Zn → 3ZnCl2 + 2Fe
Fe + H2CO3 → FeCO3 + H2 ↑
C: FeCO3 + 2HNO3 → Fe(NO3)2 + H2O+ CO2
APPEARS IN
संबंधित प्रश्न
Define the term: Vapour density.
Calculate the relative molecular mass of Sodium acetate
(use K = 39, Cl = 35.5, O = 16, C = 12, H = 1, Na = 23, N = 14, S= 32)
Calculate the relative molecular mass of Chloroform.
(use K = 39, Cl = 35.5, O = 16, C = 12, H = 1, Na = 23, N = 14, S= 32)
Fill in the blank.
Molecular weight of a gas is twice its __________.
Calculate the percentage of water in ferrous sulphate crystals.
[Fe = 56, S = 32, O =16, H = 1].
An organic compound has the following percentage composition: C = 12.76%, H = 2.13%, Br = 85.11%. The vapour density of the compound is 94. Find out its molecular formula.
Washing soda has the formula Na2CO3.10H2O.What is the mass of anhydrous sodium carbonate left when all the water of crystallization is expelled by heating 57.2 g of washing soda?
Calculate the percentage of platinum in ammonium chloroplatinate (NH4)2PtCl6.
[N = 14, H = 1, Pt = 195, Cl =35.5]
(Give your answer correct to the nearest whole number)
A flask contains 3.2g of sulphur dioxide. Calculate the following: The number of molecules of sulphur dioxide present in the flask.
Calculate the volume occupied at S.T.P. by 2 moles of SO2.
