Advertisements
Advertisements
प्रश्न
Give chemical equation of the reaction involved.
उत्तर
Chemical equation:
Zn(s) + 2HCl(aq) → ZnCl2(aq) + H2(g)
APPEARS IN
संबंधित प्रश्न
What happens when an acid reacts with a metal?
What happens when an acid reacts with a base?
What ions are present in the solutions of following substances? (write the symbols only)
Hydrochloric acid
What is the chemical formula of bleaching powder?
Which of the following salts does not contain any water of crystallisation?
Calcium phosphate is present in tooth enamel. Its nature is
In an attempt to demonstrate electrical conductivity through an electrolyte, the apparatus setup. Which among the following statement(s) is(are) correct?
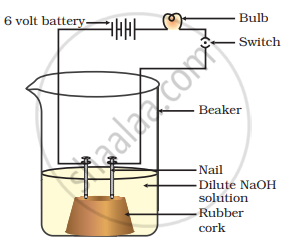
- Bulb will not glow because electrolyte is not acidic
- Bulb will glow because NaOH is a strong base and furnishes ions for conduction.
- Bulb will not glow because circuit is incomplete
- Bulb will not glow because it depends upon the type of electrolytic solution
Solutions of acids conduct ______.
______ change the colour of the indicators.
Give a balanced equation for the reaction:
Silver nitrate solution and sodium chloride solution.
