Advertisements
Advertisements
प्रश्न
Give the molecular model for a solid and use it to explain why a solid has a definite volume and a definite shape.
उत्तर
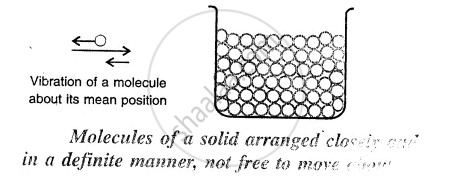
Here the molecules are very tightly packed that there is no or very less intermolecular space and there is a high intermolecular force of attraction (force of cohesion).
The molecules do not move about their mean position and thus solids have a definite shape and volume.
APPEARS IN
संबंधित प्रश्न
Water at 100°C has more heat than the steam at 100°C.
Write down five general properties of solids, liquids, and gases.
Write whether the following statement is true or false.
Intermolecular force of attraction is greater in gases than in liquids.
Write true or false for the statement. Rewrite the false statement correctly.
The intermolecular space in a gas is almost negligible.
Name the phenomenon which cause the following change.
Disappearance of camphor.
Give two examples for of the following
Substances which sublime.
Differentiate between living and non-living matter.
One kind of matter can be distinguished from another by its physical properties and chemical properties.
State what would you observe if
(a) sugar is added to pebbles take in a plastic beaker
(b) sand is added to glass balls in a beaker. What would you conclude from this imaginative demonstration?
State the property of the following substance.
Sulphur is a yellow amorphous powder insoluble in water.
