Advertisements
Advertisements
Question
Give the molecular model for a solid and use it to explain why a solid has a definite volume and a definite shape.
Solution
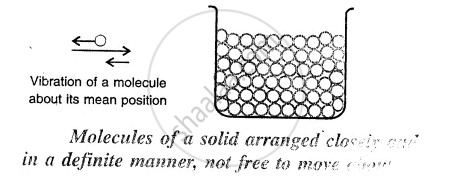
Here the molecules are very tightly packed that there is no or very less intermolecular space and there is a high intermolecular force of attraction (force of cohesion).
The molecules do not move about their mean position and thus solids have a definite shape and volume.
APPEARS IN
RELATED QUESTIONS
Evaporation takes place at ...............temperature.
Write true or false For the following Statement
The molecules in a substance arc in random motion.
How do the solids, liquids, and gases differ in their following properties.
(a) Size
(b) Shape
(c) Density
Water cycle is an example of interconversion of states of water. Explain.
Give a reason for the Pure substances have fixed melting or boiling points.
Fill in the blank
Any matter which has a definite ................. but no definite shape is called a ...............
Fill in the blank
The temperature at which a liquid boils is called the ................. point of that liquid.
Name the three states of matter and define them.
Fill in the blank with the correct word Given below.
Matter can change from one state to another by the change in ____
State the property of the following substance.
Hydrogen sulphide gas has a strong rotten egg odour.
