Advertisements
Advertisements
प्रश्न
Give one example, with equation, of the displacement of hydrogen by a metal from an acid.
उत्तर
Sodium displaces hydrogen from dilute hydrochloric acid.
2Na(s) + 2HCl(aq) → 2NaCl(aq) + H2(g)
APPEARS IN
संबंधित प्रश्न
Samples of four metals A, B, C and D were taken and added to the following solution one by one. The results obtained have been tabulated as follows.
| Metal | Iron (II) sulphate | Cooper (II) sulphate | Zinc sulphate | Silver nitrate |
| A | No reaction | Displacement | ||
| B | Displacement | No reaction | ||
| C | No reaction | No reaction | No reaction | Displacement |
| D | No reaction | No reaction | No reaction | No reaction |
Use the Table above to answer the following questions about metals A, B, C and D.
- Which is the most reactive metal?
- What would you observe if B is added to a solution of copper (II) sulphate?
- Arrange the metals A, B, C and D in the order of decreasing reactivity.
Show the formation of Na2O by the transfer of electrons.
Name the metal which has been placed at the bottom of the reactivity series.
What will happen if a strip of zinc is immersed in a solution of copper sulphate?
Complete and balance the following equation:
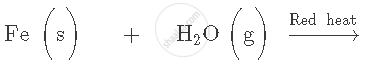
How do metals react with hydrogen? Explain with an example.
Out of the following oxides, the amphoteric oxide is:
(a) Fe2O3
(b) Al2O3
(c) P2O5
(d) N2O
What are noble gases
Write the chemical equation of the above process.
Why is acidified water considered to be a good conductor of electricity?
