Advertisements
Advertisements
Question
Give one example, with equation, of the displacement of hydrogen by a metal from an acid.
Solution
Sodium displaces hydrogen from dilute hydrochloric acid.
2Na(s) + 2HCl(aq) → 2NaCl(aq) + H2(g)
APPEARS IN
RELATED QUESTIONS
Name the following:
Metals which forms an amphoteric oxide.
Complete and balance the following equation:
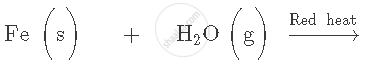
Fill in the following blank with suitable word:
Magnesium liberates ............... gas on reacting with hot boiling water.
Write the equation for the reaction of Iron with dilute hydrochloric acid.
Name the products formed. Also indicate the physical states of all the substances involved.
Out of aluminium, copper, calcium and tin, the most reactive metal is:
(a) aluminium
(b) copper
(c) tin
(d) calcium
The elements whose oxides can turn litmus solution blue are:
(a) carbon and sulphur
(b) sodium and carbon
(c) potassium and magnesium
(d) magnesium and sulphur
Metal A burns in air, on heating, to form an oxide A2O3 whereas another metal B burns in air only on strong heating to form an oxide BO. The two oxides A2O3 and BO can react with hydrochloric acid as well as sodium hydroxide solution to form the corresponding salts and water.
(a) What is the nature of oxide A2O3?
(b) What is the nature of oxide BO?
(c) Name one metal like A.
(b) Name one metal like B.
Aluminium is used for making cooking utensils. Which of the following properties of aluminium are responsible for the same?
(i) Good thermal conductivity
(ii) Good electrical conductivity
(iii) Ductility
(iv) High melting point
Which one among the following is an acidic oxide?
Find the odd one out:
