Advertisements
Advertisements
Question
Find the odd one out:
Options
Lithium (Li)
Sodium (Na)
Nitrogen (N)
Magnesium (Mg)
Solution
Nitrogen (N)
Explanation:
Because Nitrogen (N) is a non-metal.
APPEARS IN
RELATED QUESTIONS
.................... liberated when acetic acid reacts with sodium metal.
(a) Hydrogen
(b) Chlorine
(c) Oxygen
(d) Nitrogen
Name the following:
Metals which forms an amphoteric oxide.
Write equations for the reactions of calcium and potassium with water.
Explain the following reaction with the help of a balanced chemical equation:
Magnesium reacts with hot water.
What will happen if a strip of zinc is immersed in a solution of copper sulphate?
How would you show that silver is chemically less reactive than copper?
Explain why, the surface of some metals acquires a dull appearance when exposed to air for a long time.
Complete and balance the following equation:
Na + O2 →
Complete and balance the following equation:
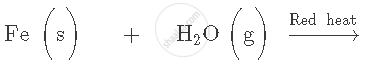
What type of oxides are formed when metals combine with oxygen? Explain with the help of an example.
What happens when magnesium reacts with very dilute nitric acid? Write an equation for the reaction involved.
Name two metals which cannot displace hydrogen from dilute acids.
The elements whose oxides can turn phenolphthalein solution pink are:
(a) Na and K
(b) K and C
(c) Na and S
(d) K and P
Out of aluminium, copper, calcium and tin, the most reactive metal is:
(a) aluminium
(b) copper
(c) tin
(d) calcium
Zinc oxide is a metal oxide. Which of the following term best describes the nature of zinc oxide:
(a) an acidic oxide
(b) a basic oxide
(c) an amphoteric oxide
(d) a neutral oxide
Metal A burns in air, on heating, to form an oxide A2O3 whereas another metal B burns in air only on strong heating to form an oxide BO. The two oxides A2O3 and BO can react with hydrochloric acid as well as sodium hydroxide solution to form the corresponding salts and water.
(a) What is the nature of oxide A2O3?
(b) What is the nature of oxide BO?
(c) Name one metal like A.
(b) Name one metal like B.
CuSO4(aq) + Fe(s) → FeSO4(aq) + Cu(s)
FeSO4(aq) + Zn(s) → ZnSO4(aq) + Fe(s)
On the basis of the above reactions, indicate which is most reactive and which is least reactive metal out of zinc, copper and iron.
Answer the following question:
An element X on reacting with oxygen forms an oxide X2O. This oxide dissolves in water and turns red litmus blue. State whether element X is metal or a non - metal. Explain with a proper example.
Which one among the following is an acidic oxide?
The substance that will be flattened on beating with a hammer is
