Advertisements
Advertisements
प्रश्न
Give reason why carbon compounds are generally poor conductors of electricity.
उत्तर
Because carbon compounds are covalent in nature, they are bad conductors of electricity; they lack free electrons.
संबंधित प्रश्न
Write structural formula of Methane.
Choose the correct answer from the options given below:
Which of the following is a common characteristic of a covalent compound?
1) high melting point
2) consists of molecules
3) always soluble in water
4) Conducts electricity when it is in the molten state
Name the element whose one of the allotropic forms is buckminsterfullerene.
Name a carbon containing molecule which has two double bonds.
What type of chemical bond is formed between carbon and bromine?
Compare the properties of ionic compounds and covalent compounds.
Why is diamond used for making cutting tools (like glass cutters) but graphite is not?
Name one covalent compound containing chlorine.
How will you find out which of the water soluble compound A or B is ionic?
The atomic numbers of four elements P, Q, R and S are 6, 10, 12 and 17 respectively. Which two elements can combine to form a covalent compound?
(a) P and R
(b) Q and S
(c) P and S
(d) R and S
One of the following compounds is not ionic in nature. This compound is:
(a) Lithium chloride
(b) Ammonium chloride
(c) Calcium chloride
(d) Carbon tetrachloride
The electronic configurations of three elements X, Y and Z are as follows:
| X | 2, 4 |
| Y | 2, 7 |
| Z | 2, 1 |
(a) Which two elements will combine to form an ionic compound?
(b) Which two elements will react to form a covalent compound?
Give reasons for your choice.
Define a covalent bond.
(a) Compound X consists of molecules.
Choose the letter corresponding to the correct answer from the choices (a), (b), (c) and (d) given below
X is likely to have a :
An element L consists of molecules.
What type of bonding is present in the particles that make up L?
Taking MgCl2 as an electrovalent compound, CCl4 as a covalent compound, give four difference between electrovalent and covalent compounds
Give an example of the covalent bond formed by
(i) Similar atoms (ii) Dissimilar atoms
Complete the following activity.
Write the names of the hydrocarbons for the following structural formula.
(isobutylene, cyclohexane, propene, cyclohexene, cyclopentane, benzene, propyne, isobutane, propane)
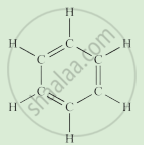 |
Considering MgCl2 as ionic compound and CH4 as covalent compound give any two differences between these two compounds.
The correct structural formula of butanoic acid is
