Advertisements
Advertisements
प्रश्न
Give reasons for the following:
Ammonolysis of alkyl halides is not a good method to prepare pure primary amines.
उत्तर
Ammonolysis of alkyl halides leads to the formation of a mixture of primary, secondary, tertiary amines and quaternary salts. It is because every time a nucleophilic substitution reaction takes place, an amine acts as a nucleophile and forms a primary amine, which further reacts and forms a secondary amine, which again reacts with the alkyl halide to form a tertiary amine and further leads to the formation of a quaternary salt. Thus, the ammonolysis reaction forms a mixture of all four compounds, and it will be difficult to get the pure amine.
\[\ce{\underset{Alkyl halide}{R-X} ->[NH3][-HX] R - NH2 ->[R-X][-HX] R2NH ->[R-X][-HX] R3N ->[R-X] R4\overset{+}{N}X^-}\]
APPEARS IN
संबंधित प्रश्न
Accomplish the following conversion:
Nitrobenzene to benzoic acid
Give the structures of A, B and C in the following reactions :

Give the structures of A, B and C in the following reactions :
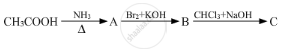
Write reactions to bring about the following conversions.
Acetamide to Ethylamine
Write reactions to bring about the following conversions.
Acetamide to methylamine
Write following conversions:
nitrobenzene `->` acetanilide
Which of the following compound gives pink colour on reaction with phthalic anhydride in cone. H2SO4 followed by treatment with NaOH?
- Phenyl methenamine
- N, N - Dimethylaniline
- N - Methyl aniline
- Benzenamine
Choose the correct order of the basic nature of the above amines.
Write a short note on the following:
Ammonolysis
Write the name of reduction product formed when ethyl cyanide is treated with sodium and alcohol.
