Advertisements
Advertisements
प्रश्न
Give reasons for the polarity of C – X bond in haloalkane.
उत्तर
- Carbon halogen bond is a polar bond as halogens are more electronegative than carbon. The carbon atom exhibits a partial positive charge (δ+) and halogen atom a partial negative charge (δ–).
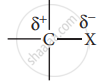
- The C -X bond is formed by the overlap of sp3 orbital of a carbon atom with the half-filled p- orbital of the halogen atom. The atomic size of halogen increases from fluorine to iodine, which increases the C – X bond length. Larger the size, greater is the bond length, and the weaker is the bond formed. The bond strength of C – X decreases from C – F to C – I in CH3X.
APPEARS IN
संबंधित प्रश्न
Benzene reacts with Cl2 in the presence of FeCl3 and in absence of sunlight to form ______.
The most easily hydrolysed molecule under SN1 condition is ______.
Which one of the following is most reactive towards nucleophilic substitution reaction?
t – butyl chloride reacts with aqueous KOH by SN1 mechanism while n – butyl chloride reacts with SN2 mechanism.
p – dichlorobenzene has a higher melting point than those of o – and m – dichlorobenzene.
In an experiment ethyliodide in ether is allowed to stand over magnesium pieces. Magnesium dissolves and product is formed
- Name the product and write the equation for the reaction.
- Why all the reagents used in the reaction should be dry? Explain.
- How is acetone prepared from the product obtained in the experiment.
Predict the products when Bromo ethane is treated with the following.
AgNO2
Write a short note on Dows Process.
Starting from CH3MgI, How will you prepare the following?
Iso propyl alcohol
Complete the following reaction.
\[\ce{CH3 - CH = CH2 + HBr ->[Peroxide]}\]
