Advertisements
Advertisements
प्रश्न
How is ammonia manufactured industrially?
उत्तर १
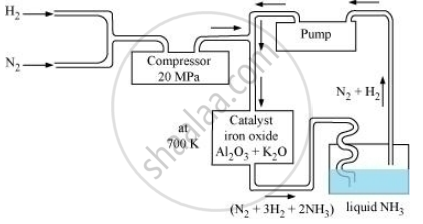
Ammonia is prepared on a large-scale by the Haber’s process.
`N_2(g) + 3H_2(g) ⇌ 2NH_3(g) triangle_fH^@ = -46.1 kJmol`
The optimum conditions for manufacturing ammonia are:
(i) Pressure (around 200 × 105 Pa)
(ii) Temperature (700 K)
(iii) Catalyst such as iron oxide with small amounts of Al2O3 and K2O
उत्तर २
Commercially, by Haber’s process.
`N_2(g) + 3H_2(g) ⇌ 2NH_3(g) triangle_fH^@ = -46.1 kJ mol^(-1)`
iron oxide, K2O, Al203 The optimum conditions for the production of NH3 are pressure of 200 atm and temperature of 100K
APPEARS IN
संबंधित प्रश्न
Describe the laboratory method of preparation of ammonia
What is the action of Excess of air on ammonia ?
What is the action of the following reagents on ammonia :
Nessler's reagent
How does ammonia react with a solution of Cu2+?
What is the action of Excess of chlorine on ammonia?
What is the action of Na Metal on ammonia?
What is the action of the following reagents on ammonia :
Sodium metal
Which compound is used in the preparation of caprolactam?
Which compound is used as the cooling liquid in refrigerators?
Ammonia on reaction with hypochlorite anion can form:
When ammonia is heated with cupric oxide, a molecule of ammonia will ____________.
Ammonia is generally manufactured for fertilizers by the reaction:
In qualitative analysis when \[\ce{H2S}\] is passed through an aqueous solution of salt acidified with dil. \[\ce{HCl}\], a black precipitate is obtained. On boiling the precipitate with dil. \[\ce{HNO3}\], it forms a solution of blue colour. Addition of excess of aqueous solution of ammonia to this solution gives ______.
A brown ring is formed in the ring test for \[\ce{NO3^{-}}\] ion. It is due to the formation of ______.
In the preparation of HNO3, we get NO gas by catalytic oxidation of ammonia. The moles of NO produced by the oxidation of two moles of NH3 will be ______.
What happens when reactions:
Benzylchloride is treated with ammonia followed by the reaction with Chloromethane.
