Advertisements
Advertisements
प्रश्न
How can propan-2-one be converted into tert- butyl alcohol?
उत्तर
\[\begin{array}{cc}
\phantom{...........}\ce{O}\phantom{................................}\ce{CH3}\phantom{.................}\ce{CH3}\phantom{.....................}\\
\phantom{............}||\phantom{.................................}|\phantom{.....................}|\phantom{.........................}\\
\phantom{}\ce{CH3 - C - CH3 + CH3 MgI ->[Ether] CH3 - C - CH3 ->[H+][H2O] CH3 - C - CH3 + Mg(I)OH}\phantom{.}\\
\phantom{......................}|\phantom{.....................}|\phantom{.}\\
\phantom{.........................}\ce{\underset{Addition product}{CH3}}\phantom{.........}\ce{\underset{\underset{(2-methylpropan-2-ol)}{tert-Butyl alcohol}}{OH}}\phantom{.}
\end{array}\]
APPEARS IN
संबंधित प्रश्न
Name the reagents used in the following reactions:
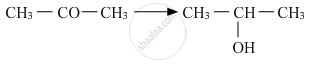
Show how is the following alcohol prepared by the reaction of a suitable Grignard reagent on methanal?
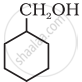
Show how you would synthesise the following alcohol from an appropriate alkene?
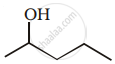
Show how you would synthesise the following alcohol from an appropriate alkene?
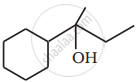
Name the reagents used in the following reactions:

Benzaldehyde differs from acetaldehyde in that:
Acetone reacts with Grignard reagent to form:
The major product of acid catalysed dehydration of 1-methylcyclohexanol is ______.
\[\ce{C3H8O ->[{[O]}][K2Cr2O7/H2SO4] C3H6O ->[I2 + NaOH(aq.)] CHI3}\]
In this reaction the first compound is:
Write the mechanism of acid dehydration of ethanol to yield ethene.
