Advertisements
Advertisements
प्रश्न
iii) Observe the figure and answer the following questions.
a) Identify the block shown by box A and write an electronic configuration of any one element of this block.
b) Identify the block of element denoted by letter B and write its period number.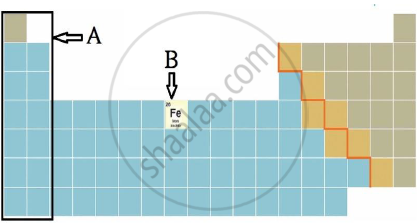
उत्तर
a) The block shown by box A is s-Block.
S(16) = 2, 8, 6 (electronic configuration of any element in s block)
b) The block of element denoted by letter B is d – Block.
The period number of that element is 4.
APPEARS IN
संबंधित प्रश्न
Name any five periods properties.
Explain the following:
Group 17 elements are strong non-metals, while group 1 elements are strong metals.
How do the following change on moving from left to right in a period of the periodic table?
Give examples in support of your answer.
Nature of oxides of the elements ?
How does the chemical reactivity of
alkali metals vary?
The electronic configuration of an element T is 2, 8, 8, 1.
Is it a metal or a non-metal?
Complete the following sentences
Moving across a ………….. of the periodic table the elements show increasing ………………..character (group, period, metallic, non-metallic).
A metal M forms as oxide having the formula M2O3. It belongs to third period. Write the atomic number and valency of the metal.
Explain why the following statement is not correct:
All groups contain metals and non metals.
Study the extract of the Periodic Table given below and answer the questions that follow. Give the alphabet corresponding to the element in question. DO NOT repeat an element. 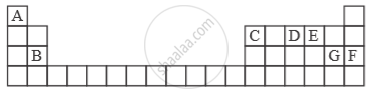
Which non-metallic element has the valency of 2?
For the main group of the periodic table, the metallic properties of the elements vary approximately with their position as shown in the table.
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
| H | He | ||||||
| A | B | ||||||
| C | D |
Will the most metallic element be found at A,B,C or D ?
State whether the following statement is true or false
All members of zero group are non metals.
In the third period, which is the most metallic and most non-metallic element?
If an element is in group 7 is it likely to be metallic or non metallic in character?
Copy and complete the following sentence choosing the correct word or words from those given below, at the end of the sentence:
The element at the bottom of a group would be expected to show ______ metallic character than the element at the top.
Within a group, where would you expect to find the element with the greatest metallic character.
Arrange the following elements in order of their decreasing metallic character.
Na, Si, Cl, Mg, Al
Write the name.
The atom having the smallest atomic radius from zero group.
Write the name.
Two elements having two orbits.
Identify the elements with the following property and arrange them in increasing order of their reactivity
- An element which is a soft and reactive metal
- The metal which is an important constituent of limestone
- The metal which exists in liquid state at room temperature
