Advertisements
Advertisements
प्रश्न
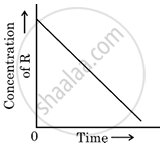
In a given graph of zero-order reaction, the slope and intercept are:
पर्याय
Slope = k, Intercept = [R]0
Slope = −k, Intercept = [R]0
Slope = `k/2.303`, Intercept = In[R]0
Slope = `−k/2.303`, Intercept = In A
MCQ
उत्तर
Slope = −k, Intercept = [R]0
Explanation:
According to the integrated rate law for zero order:
[R] = −kt + [R0]
So the slope is equal to the negative of rate constant −k and the intercept is equal to the initial concentration R0.
shaalaa.com
या प्रश्नात किंवा उत्तरात काही त्रुटी आहे का?
