Advertisements
Advertisements
प्रश्न
In the SF4 molecule, there are:
पर्याय
4 lone pairs and one bond pair and shape is tetrahedral
4 bond pairs and one lone pair and shape is see-saw
2 lone pairs and four bond pair and shape is linear
1 lona pairs and 6 bond pair and shape is octahedral
MCQ
उत्तर
4 bond pairs and one lone pair and shape is see-saw
Explanation:
No. of bond pair: 4
No. of lone pair: 1
Shape - See saw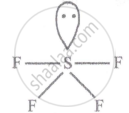
SF4 (sp3d hyb.)
shaalaa.com
Close Packed Structures of Solids
या प्रश्नात किंवा उत्तरात काही त्रुटी आहे का?
