Advertisements
Advertisements
प्रश्न
In which of the following pairs, the two species are isostructural:
पर्याय
\[\ce{SO^{2-}_3}\] and \[\ce{NO^-_3}\]
BF3 and NF3
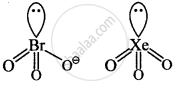
\[\ce{BrO^-_3}\] and \[\ce{XeO3}\]
SF4 and XeF4
MCQ
उत्तर
\[\ce{BrO^-_3}\] and \[\ce{XeO3}\]
Explanation:
Both are pyramidal.
shaalaa.com
या प्रश्नात किंवा उत्तरात काही त्रुटी आहे का?
