Advertisements
Advertisements
प्रश्न
Justify the following reaction as a redox reaction.
\[\ce{2Na_{(s)} + S_{(s)}->Na2S_{(s)}}\]
Find out the oxidizing and reducing agents.
उत्तर
- Redox reaction can be described as electron transfer, as shown below:
\[\ce{2Na_{(s)} + S_{(s)}->2Na^+ + S^2-}\] - Charge development suggests that each sodium atom loses one electron to form \[\ce{Na+}\] and sulphur atom gains two electrons to form S2–. This can be represented as follows:
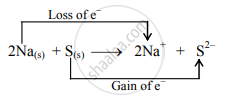
- When Na is oxidized to Na2S, the neutral Na atom loses electrons to form Na+ in Na2S while the elemental sulphur gains electrons and forms S2– in Na2S.
- Each of the above steps represents a half-reaction which involves electron transfer (loss or gain).
- Sum of these two half-reactions or the overall reaction is a redox reaction.
- Oxidizing agent is an electron acceptor and hence, S is an oxidizing agent. Reducing agent is an electron donor and hence, Na is a reducing agent.
APPEARS IN
संबंधित प्रश्न
Choose the correct option.
The process \[\ce{SO2->S2Cl2}\] is
In which reaction does nitrogen exhibit variation of oxidation state from –3 to +5?
Calculate the oxidation number of the underlined atom.
H3PO3
Calculate the oxidation number of the underlined atom.
K2C2O4
Identify the following pair of species is in its oxidized state?
Cu/Cu2+
Identify the following pair of species is in its oxidized state?
\[\ce{O2/O^2-}\]
Identify the following pair of species is in its oxidized state?
Cl2/Cl−
Provide the stock notation for the following compound:
HAuCl4
Provide the stock notation for the following compound:
FeO
Assign oxidation number atom in the following species.
\[\ce{Cr(OH)^Θ_4}\]
Assign oxidation number atom in the following species.
Na2S2O3
Assign oxidation number atom in the following species.
H3BO3
Which of the following redox couple is a stronger oxidizing agent?
\[\ce{MnO^Θ_4}\](E0 = 1.51 V) and \[\ce{Cr2O^{2Θ}_7}\](E0 = 1.33 V)
The following statements are CORRECT, EXCEPT:
Oxidation state of Xe in XeOF4 is ____________.
The oxidation number of oxygen in peroxides is ____________.
Which of the following is NOT an example of redox reaction?
Identify the strongest oxidising agent.
\[\ce{Na^+ + e^- -> Na}\]; E0 = −2.714 V
\[\ce{Pt^{2+} + 2e^- -> Pt}\]; E0 = +1.200 V
\[\ce{I2 + 2e^- -> 2I^-}\]; E0 = + 0.535 V
\[\ce{Co^{2+} + 2e^- -> Co}\]; 0 = −0.280 V
The oxidation number of sulphur in S8 molecule is ______.
Match the following.
| Compound | Oxidation no. of underlined element |
| i. \[\ce{\underline{C}_4H4O^{2-}_6}\] | a. +2.5 |
| ii. \[\ce{\underline{N}_3H}\] | b. +1.5 |
| iii. \[\ce{Mg2\underline{P}_2O7}\] | c. +5 |
| iv. \[\ce{Na2\underline{S}_4O6}\] | d. `-1//3` |
In which of the following, oxidation number of oxygen is +2?
All of these are CORRECT for the complex K4[Mn(CN)6], EXCEPT:
When methane undergoes combustion in air, the oxidation number of C ____________.
The sum of oxidation states of all atoms in \[\ce{Cr2O^{2-}_7}\] ion is ______.
The oxidation state of phosphorous in Mg2P2O7 is ______.
The sum of oxidation number of all atoms in \[\ce{SnO^{2-}_3}\] ion is _______.
What is the oxidation state of chlorine atom in perchloric acid?
