Advertisements
Advertisements
Question
Justify the following reaction as a redox reaction.
\[\ce{2Na_{(s)} + S_{(s)}->Na2S_{(s)}}\]
Find out the oxidizing and reducing agents.
Solution
- Redox reaction can be described as electron transfer, as shown below:
\[\ce{2Na_{(s)} + S_{(s)}->2Na^+ + S^2-}\] - Charge development suggests that each sodium atom loses one electron to form \[\ce{Na+}\] and sulphur atom gains two electrons to form S2–. This can be represented as follows:
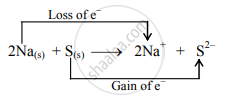
- When Na is oxidized to Na2S, the neutral Na atom loses electrons to form Na+ in Na2S while the elemental sulphur gains electrons and forms S2– in Na2S.
- Each of the above steps represents a half-reaction which involves electron transfer (loss or gain).
- Sum of these two half-reactions or the overall reaction is a redox reaction.
- Oxidizing agent is an electron acceptor and hence, S is an oxidizing agent. Reducing agent is an electron donor and hence, Na is a reducing agent.
APPEARS IN
RELATED QUESTIONS
Choose the correct option.
Oxidation numbers of Cl atoms marked as Cla and Clb in CaOCl2 (bleaching powder) are
\[\begin{array}{cc} \ce{Cl^{{a}}}\phantom{.} \\/\phantom{...} \\ \ce{Ca}\phantom{......} \\ \backslash\phantom{..} \\\phantom{........} \ce{O-Cl^{{b}}}\phantom{.} \end{array}\]
Choose the correct option.
A compound contains atoms of three elements A, B, and C. If the oxidation state of A is +2, B is +5 and that of C is -2, the compound is possibly represented by
Choose the correct option.
Which is the correct stock notation for manganese dioxide?
Choose the correct option.
Oxidation number of oxygen in superoxide is
In which reaction does nitrogen exhibit variation of oxidation state from –3 to +5?
Calculate the oxidation number of the underlined atom.
H2S4O6
What is oxidation?
Identify the following pair of species is in its oxidized state.
Mg/Mg2+
Provide the stock notation for the following compound:
HAuCl4
Provide the stock notation for the following compound:
Tl2O
Provide the stock notation for the following compound:
Fe2O3
Assign oxidation number atom in the following species.
H3BO3
Which of the following redox couple is a stronger oxidizing agent?
Cl2 (E0 = 1.36 V) and Br2 (E0 = 1.09 V)
The following statements are CORRECT, EXCEPT:
The oxidation number of oxygen in peroxides is ____________.
What is the oxidation number of As in H3AsO3?
Which of the following is NOT an example of redox reaction?
In the complex [Co(en)3]Cl3, ____________.
In the following reaction, the oxidation number of Cr changes.
\[\ce{ClO^-_{( aq)} + Cr(OH)^-_{4(aq)} -> CrO^{2-}_{4(aq)} + Cl^-_{( aq)} (basic)}\]
In which of the following, oxidation number of oxygen is +2?
All of these are CORRECT for the complex K4[Mn(CN)6], EXCEPT:
Which of the following is INCORRECT?
The sum of oxidation states of all atoms in \[\ce{Cr2O^{2-}_7}\] ion is ______.
What is the oxidation number of Carbon in K2C2O4?
The oxidation state of phosphorous in Mg2P2O7 is ______.
The oxidation number of Cr in \[\ce{Cr(OH)^-_4}\] ion is ______.
The oxidation number of phosphorous in Ba(H2PO2)2 is ______.
The brown ring complex is formulated as [Fe(H2O)5NO]SO4. The oxidation number of iron is ______.
The oxidation number of oxygen in oxygen difluoride (OF2) and dioxygen difluoride (O2F2) respectively is ______.
