Advertisements
Advertisements
प्रश्न
Mention three important uses of nitric acid. Give the property of nitric acid involved in the use.
उत्तर
Three important uses of Nitric acid and the property of nitric acid involved is:
| Sl. NO. | Use | Property |
| 1 | To etch designs on copper and brassware. | Nitric acid act as solvent for large number of metals. |
| 2 | To purify gold. | Impurities like Cu, Ag, Zn, etc. dissolve in nitric acid. |
| 3 | Preparation of aqua regia. | Dissolves noble metals. |
APPEARS IN
संबंधित प्रश्न
Name the products formed when dilute \[\ce{HNO3}\] is added to copper.
Explain with the help of a balanced equation, the brown ring test for nitric acid.
Explain with the help of a balanced equation, the brown ring test for nitric acid.
Choose the correct answer from the option given below:
The catalyst used in the manufacture of HNO3 by Ostwald process is
Fill in the blank with appropriate word/words.
Aqua regia is a mixture of _______ and________
Give reason for the following:
Commercial concentrated nitric acid is yellow in colour, but when it is dilute with water, it turns colourless.
Give one chemical test for nitric acid.
Name the compounds required for the laboratory preparation of nitric acid.
The figure given below illustrates the apparatus used in the laboratory preparation of nitric acid: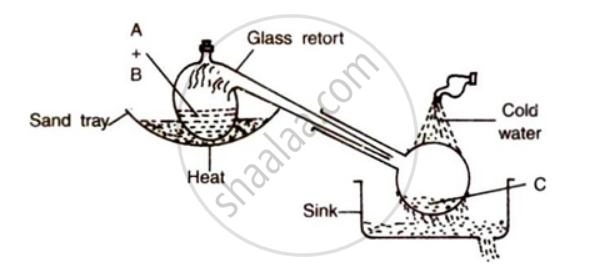
(i) Name A (a liquid), B( a Solid) and C(a liquid).
(ii) write the balanced chemical equation to show how nitric acid undergoes decomposition.
(iii) Write the balanced chemical equation for the reaction in which copper is oxidized by concentrated nitric acid.
Balanced equation of oxidation of carbon with concentrated HNO3
Write a balanced equation for following :
Laboratory preparation nitric acid.
