Advertisements
Advertisements
प्रश्न
Name of physical quantity which remains same for microwaves of wavelength 1 mm and UV radiations of 1600 Å in vacuum.
उत्तर
Both microwaves and UV rays are a part of the electromagnetic spectrum. Thus, the physical quantity that remains same for both types of radiation will be their speeds, equal to c.
APPEARS IN
संबंधित प्रश्न
A plane electromagnetic wave travels in vacuum along z-direction. What can you say about the directions of its electric and magnetic field vectors? If the frequency of the wave is 30 MHz, what is its wavelength?
Use the formula λm T= 0.29 cm K to obtain the characteristic temperature ranges for different parts of the electromagnetic spectrum. What do the numbers that you obtain tell you?
What is the range of the wavelength of the following electromagnetic waves?
(a) Micro waves .
Give one use of electromagnetic radiation in Ultraviolet radiation.
The frequency of x-rays, y-rays and ultraviolet rays are respectively a, b and c. Then:-
The ozone layer absorbs
Following QN ∴ 14, the radiation force on the roof will be
The frequency of e. m waves which is best suited .to observed of radius 3 × 10–4 his of the order of
Name one radiation having the wavelength longer than the wavelength of these radiations.
In an atom X, electrons absorb the energy from an external source. This energy “excites” the electrons from a lower-energy level to a higher-energy level around the nucleus of the atom. When electrons return to the ground state, they emit photons.
The figure below is the energy level diagram of atom X with three energy levels, E1 = 0.00eV, E2 = 1.78eV and E3 = 2.95eV. The ground state is considered 0 eV for reference. The transition of electrons takes place between levels E1 and E2.
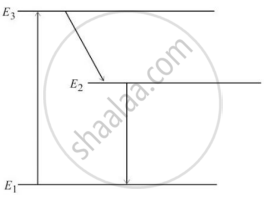
- What wavelength of radiation is needed to excite the atom to energy level E2 from E1?
- Suppose the external source has a power of 100 W. What would be the rate of photon emission?
