Advertisements
Advertisements
प्रश्न
With what neutral molecule is ClO− isoelectronic? Is that molecule a Lewis base?
उत्तर १
ClO− is isoelectronic to ClF. Also, both species contain 26 electrons in all as shown.
Total electrons ClO− = 17 + 8 + 1 = 26
In ClF = 17 + 9 = 26
ClF acts like a Lewis base as it accepts electrons from F to form ClF3.
उत्तर २
CIO– has 17 + 8 +1 = 26 electrons.Also, OF2 has (8 + 2 x 9) = 26 electrons, and ClF has (17 + 9) = 26 electrons.Out of these, ClF can act as Lewis base. The chlorine atom has three lone pair of electrons which it donates to form compounds like ClF3, ClF5 and ClF7
APPEARS IN
संबंधित प्रश्न
Account for the following: Fluorine does not exhibit positive oxidation state.
Account for the following :
Acidic character increases from HF to HI.
Which halogen compound in each of the following pairs will react faster in SN2 reaction CH3Br or CH3I
Give two examples to show the anomalous behaviour of fluorine.
Complete the following equations:
KMnO4 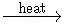
Arrange the following in the decreasing order of their reducing character :
HF, HCl, HBr, HI
Select the correct alternative from the choices given :
The correct order of increasing acidic strength of the oxoacids of chlorine is:
Cl2 acts as a bleaching agent.
Tincture of iodine is ____________.
Which one has the lowest boiling point?
Bond dissociation enthalpy of E–H (E = element) bonds is given below. Which of the compounds will act as the strongest reducing agent?
| Compound | \[\ce{NH3}\] | \[\ce{PH3}\] | \[\ce{AsH3}\] | \[\ce{SbH3}\] |
| Δdiss (E – H)/kJ mol–1 | 389 | 322 | 297 | 255 |
Which of the following is isoelectronic pair?
Solubility of iodine in water may be increase by adding
The reaction \[\ce{3ClO- (aq) -> ClO3- (aq) + 2Cl- (aq) + 2Cl- (aq)}\] is an example of
Iodine has lowest oxidiation state in ______.
Statement I: Acid strength increases in the order given as HF << HCl << HBr << HI.
Statement II: As the size of the elements F, Cl, Br, and I increase down the group, the bond strength of HF, HCl, HBr, and HI decreases and so the acid strength increases.
In the light of the above statements, choose the correct answer from the options given below.
Write the name and formula of oxoacid of fluorine.
Give a reason for the following:
Mn+2 compounds are more stable than Fe+2 compounds.
