Advertisements
Advertisements
प्रश्न
Observe the following diagram and give answers.
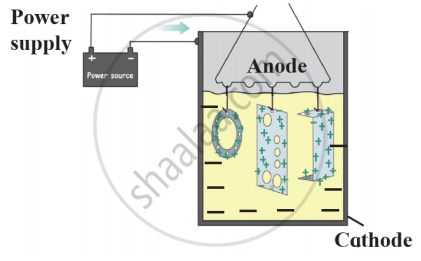
- Name this method of prevention of corrosion.
- For prevention of which metal this method is used?
- What is used as anode in this method?
उत्तर
- The given diagram represents anodizing process used for the prevention of corrosion.
- This method is used for the prevent corrosion of aluminium and copper.
- In this method, copper or aluminium article is used as the anode.
APPEARS IN
संबंधित प्रश्न
Give two examples of alloys with their chemical composition.
Tinning : Tin : : Galvanizing : _________
Which of the following methods is suitable for preventing an iron frying pan from rusting?
Name any three objects (or structures) which are gradually damaged by the corrosion of iron and steel.
Name the metal which is used for galvanising iron.
Explain why, iron sheets are coated with zinc.
Fill in the following blank with suitable word:
The corrosion of copper produces a .............. coating of basic copper carbonate on its surface
Why is an iron grill painted frequently?
What is corrosion?
Name any two metals which do not corrode easily.
What is meant by 'rusting of iron'? With the help of labelled diagrams, describe an activity to find out the conditions under which iron rusts.
Name two metals which resist corrosion due to the formation of a thin, hard and impervious layer of oxide on their surface.
In stainless steel alloy, iron metal is mixed with:
(a) Cu and Cr
(b) Cr and Ni
(c) Cr and Sn
(d) Cu and Ni
Brass is an alloy of:
(a) Cu and Sn
(b) Cu and Pb
(c) Pb and Sn
(d) Zn and Cu
Name the metal which is a constituent of plant pigment?
Explain how the activity series accounts for each of the following:
tendency to corrosion
Explain with reason:
Roasting is carried out on sulphide ores and not on carbonate ores.
Observe the following picture a write down the chemical reaction with the explanation.

What is done to prevent corrosion of metals?
Answer the following question.
List two properties of alloys.
Give reason.
A wooden article should be polished.
Explain the term – rusting and give a word equation for the formation of rust. If polished iron nails are kept in three separate test tubes, state the contents in each test tube required, to prove the conditions for rusting.
State whether the statement given below is true or false. If false write the correct statement.
Either oxygen or moisture is essential for rusting.
Corrosion of silver causes a black layer of _______.
_______ forms a green colour in the water.
Find the odd one out and give its explanation.
Observe the following figure and write the answer of the question.

- Which process is shown in the figure?
- Explain the chemical reaction shown in the figure.
- Write the reactions on anode and cathode.
What is rust?
The diagram shows the reaction between metal and dil. acid.
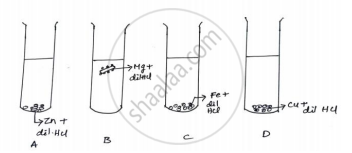
What is the reason for different behaviour of Mg in test tube B?
A man painted his main gate made up of iron, to
- prevent it from rusting.
- protect it from the sun.
- make it look beautiful.
- make it dust-free.
Which of the above statement(s) is/are correct?
Give an example of a chemical reaction for each of the following situations:
Sound is produced
Gold plated ornaments is the example of ______.
In ______ process a layer of molten tin is deposited on metals.
Describe two changes that are harmful. Explain why you consider them harmful. How can you prevent them?
