Advertisements
Advertisements
प्रश्न
Why is an iron grill painted frequently?
उत्तर
To prevent rusting of iron substances, they are painted frequently. Iron is a reactive metal and it starts corroding in the presence of air (oxygen) and water (or moisture). Iron reacts with oxygen present in the moist air and forms a brown flaky iron oxide (or rust) layer on its surface. This is called rusting. This rusting causes huge damage to iron substances by making them weak. Therefore, an iron grill is painted frequently to prevent corrosion.
APPEARS IN
संबंधित प्रश्न
Which metals do not corrode easily?
What is anodising? Give its applications.
The chemical reaction involved in the corrosion of iron metal is that of:
(a) oxidation as well as displacement
(b) reduction as well as combination
(c) oxidation as well as combination
(d) reduction as well as displacement
Fill in the following blank with suitable word:
The process of depositing a thin layer of zinc on iron articles is called .............
What is meant by 'rusting of iron'? With the help of labelled diagrams, describe an activity to find out the conditions under which iron rusts.
State under what conditions corrosion is faster ?
Corrosion can be an advantage in some case.Explain ?
Identify the process shown in the diagram and explain it in short
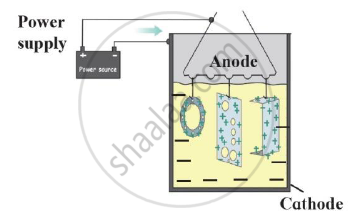
Answer the following question.
a) What is meant by corrosion?
b) Write names of any two methods of prevention of corrosion.
c) In which method, metal like copper, aluminium are coated with a thin layer of their oxides by means of electrolysis.
d) Explain this method with diagram.
Give reason.
An iron article should be given a coat of paint
Give reason.
A wooden article should be polished.
Explain the term – rusting and give a word equation for the formation of rust. If polished iron nails are kept in three separate test tubes, state the contents in each test tube required, to prove the conditions for rusting.
State whether the statement given below is true or false. If false write the correct statement.
Graphite is a lustrous non-metal which conducts electricity.
_______ forms a green colour in the water.
Bronze : _______ : : Stainless steel : Fe + Cr + C
Find the odd one out and give its explanation.
Find the odd one out and give its explanation.
Draw a neat labelled diagram.
Anodizing
Alloys are homogeneous mixtures of a metal with a metal or nonmetal. Which among the following alloys contain non-metal as one of its constituents?
