Advertisements
Advertisements
प्रश्न
Out of C and CO, which is a better reducing agent for ZnO ?
उत्तर १
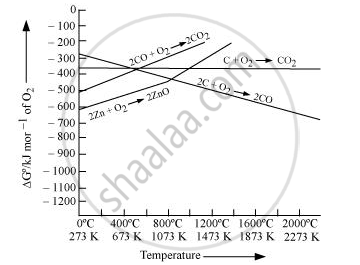
Reduction of ZnO to Zn is usually carried out at 1673 K. From the above figure, it can be observed that above 1073 K, the Gibbs free energy of formation of CO from C and above 1273 K, the Gibbs free energy of formation of CO2 from C is lesser than the Gibbs free energy of formation of ZnO. Therefore, C can easily reduce ZnO to Zn.
On the other hand, the Gibbs free energy of formation of CO2 from CO is always higher than the Gibbs free energy of formation of ZnO. Therefore, CO cannot reduce ZnO. Hence, C is a better reducing agent than CO for reducing ZnO.
उत्तर २
The two reduction reactions are :
`ZnO(s) + C(s) -> Zn(s) + CO(g)` ... (i)
`ZnO(s) + CO(g) -> Zn(s) + CO_2(g)` .... (ii)
In the first case, there is increase in the magnitude of ΔS° while in the second case, it almost remains the same. In other words ΔG° will have more negative value in the first case when C(s) is used as the reducing agent than in the second case when CO(g) acts as the reducing agent. Therefore, C(s) is a better reducing agent.
APPEARS IN
संबंधित प्रश्न
Out of C and CO, which is a better reducing agent at 673 K?
Giving examples, differentiate between ‘roasting’ and ‘calcination’.
Why copper matte is put in silica lined converter?
Which one of the following reaction represents calcinations?
Flux is a substance which is used to convert
Zinc is obtained from ZnO by ____________.
What is the role of Limestone in the extraction of iron from its oxide Fe2O3?
Explain the following term with a suitable example.
Slag
Which reagents are required for one step conversion of chlorobenzene to toluene?
At the temperature corresponding to which of the points in figure, FeO will be reduced to Fe by coupling the reaction \[\ce{2FeO -> 2Fe + O2}\] with all of the following reactions?
(a) \[\ce{C + O2 -> CO2}\]
(b) \[\ce{2C + O2 -> 2CO}\]
(c) \[\ce{2CO + O2 -> 2CO2}\]
(i) Point A
(ii) Point B
(iii) Point D
(iv) Point E
Pb and Sn are extracted from their chief are by:-
CN– solution is used in the extraction of which metal?
Colemanite is:-
Sulphide ore of the metal are usually concentrated by froth floatation process. Which of the following sulphide ore offers an exception and is concentrated by chemical leaching?
Heating byrites to remove sulphtir is called
