Advertisements
Advertisements
प्रश्न
Schottky defect is observed in crystals when ______.
पर्याय
some cations move from their lattice site to interstitial sites.
equal number of cations and anions are missing from the lattice.
some lattice sites are occupied by electrons.
some impurity is present in the lattice.
उत्तर
Schottky defect is observed in crystals when equal number of cations and anions are missing from the lattice.
Explanation:
| Defect | Definition | Type of solid |
Effect of defect on density of substance |
Structure of crystal (with defect) |
| Schottky defect |
A crystal is said to have Schottky defect if equal number of cations and anions are missing from their normal lattice site there by creating vacancies or holes. |
Ionic solid |
Decreases | 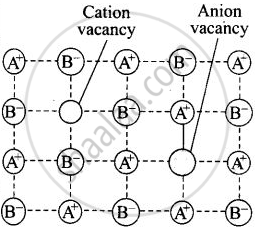 Schottky defect in a crystal |
APPEARS IN
संबंधित प्रश्न
Explain the following terms with suitable examples: Frenkel defect
Defects in solids can be studied using
Schottky defect defines imperfection in the lattice structure of ____________.
Assertion: No compound has both Schottky and Frenkel defects.
Reason: Both defects change the density of the solid.
Which of the following crystals does not exhibit Frenkel defect?
Silver halides generally show:
What is the effect of Frenkel defect on the density of ionic solids?
In a Schottky defect ____________.
Which of the following defects decrease the density?
(i) Interstitial defect
(ii) Vacancy defect
(iii) Frankel defect
(iv) Schottky defect
Given below are two statements, one is labelled as Assertion (A) and the other is labelled as Reason (R):
Assertion: In any ionic solid (MX) with Schottky defects, the number of positive and negative ions are same.
Reason: Equal number of cation and anion vacancies are present.
