Advertisements
Advertisements
प्रश्न
State your observation for the following case :
Dry red rose petals are placed in the jar of sulphur dioxide.
उत्तर
Dry SO2 has no effect on dry red rose petals.
APPEARS IN
संबंधित प्रश्न
Write a balanced chemical equation for the following:
Action of concentrated sulphuric acid on Sulphur.
Which property of sulphuric acid is shown by the reaction of the concentrated sulphuric acid with Carbon?
Give a chemical test to distinguish between dilute sulphuric acid and conc. sulphuric acid.
Give reason for the following:
When concentrated sulphuric acid is exposed to air, its volume increases and it becomes slightly dilute.
The following statement is correct only under certain conditions. Rewrite the statement including the appropriate conditions.
Oxalic acid reacts with sulphuric acid to produce carbon monoxide and carbon dioxide.
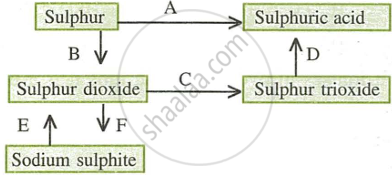
- Name the catalyst which helps in the conversion of sulphur dioxide to sulphur trioxide in step C.
- In the contact process for the manufacture of sulphuric acid, sulphur trioxide is not converted to sulphuric acid by reacting it with water. Instead a two-step procedure is used. Write the equations for the two steps involved in D.
- What type of substance will liberate sulphur dioxide from sodium sulphite in step E?
- Write the equation for the reaction by which sulphur dioxide is converted to sodium sulphite in step F.
What property of sulphuric acid is shown by the reaction of concentrated sulphuric acid when heated with :
(i) Potassium nitrate
(ii) carbon
In the given equation identify the role played by concentrated sulphuric acid S + 2H2SO4→ 3SO2 + 2H2O
Give one equation to show the following property of sulphuric acid:
Acidic nature
Write the balanced chemical equation for the following conversion:
Lead sulphate from lead nitrate solution and dilute sulphuric acid.
