Advertisements
Advertisements
प्रश्न
Structure of the compound whose IUPAC name is 5, 6 – dimethylhept - 2 - ene is
पर्याय
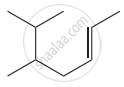
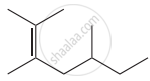
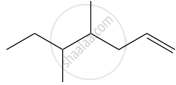
None of these
उत्तर

APPEARS IN
संबंधित प्रश्न
Draw a formula for the first five members of the homologous series beginning with the given compound.
H–CH=CH2
An organic compound containing carbon, hydrogen and oxygen is soluble in dil. H2SO4 and does not react with sodium metal or KMnO4. When it is heated with HI in excess, a single alkyl halide is obtained. The original compound can be ____________.
How many primary, secondary and tertiary carbon atoms respectively are present in isobutane?
An organic compound 'X' (molecular formula C6H7O2N) has six carbons in a ring system, two double bonds and also a nitro group as a substituent, 'X' is ______.
The correct decreasing order of priority for the functional groups of organic compounds in the IUPAC system of nomenclature is ______.
Identify primary, secondary, tertiary, and quaternary carbon in the following compound.
\[\begin{array}{cc}
\ce{CH3}\phantom{..................}\\
|\phantom{....................}\\
\ce{CH3 - C - CH - CH2 - CH2 - CH3}\\
|\phantom{....}|\phantom{................}\\
\ce{CH3} \ce{CH3}\phantom{..............}
\end{array}\]
Identify the α - carbons in the following species and give the total number of
\[\ce{CH3 -CH2 -\overset{⊕}{C}H -CH2 -CH3}\]
Identify primary, secondary, tertiary, and quaternary carbons in the following compound.
\[\begin{array}{cc}
\ce{CH3\phantom{.................}}\\
|\phantom{....................}\\
\ce{CH3 - C - CH - CH2 - CH2 - CH3}\\
|\phantom{....}|\phantom{................}\\
\ce{CH3 CH3\phantom{.............}}
\end{array}\]
Identify primary, secondary, tertiary and quaternary carbon in the following compound.
\[\begin{array}{cc}
\ce{CH3}\phantom{..................}\\
|\phantom{....................}\\
\ce{CH3 - C - CH - CH2 - CH2 - CH3}\\
|\phantom{....}|\phantom{................}\\
\ce{CH3}\ce{CH3}\phantom{..............}
\end{array}\]
Identify the α-carbons in the following species and give the total number of α-hydrogen.
\[\ce{CH2 = CH - CH2 - CH3}\]
