Advertisements
Advertisements
प्रश्न
The element below sodium in the same group would be expected to have a ______ (lower/higher) electro-negativity than sodium and the element above chlorine would be expected to have a ______ (lower/higher) ionization potential than chlorine.
उत्तर
The element below sodium in the same group would be expected to have a lower electro-negativity than sodium, and the element above chlorine would be expected to have a higher ionisation potential than chlorine.
APPEARS IN
संबंधित प्रश्न
Choose the most appropriate answer of the following:
Among the elements given below, the element with the least electronegativity is:
(A) Lithium
(B) Carbon
(C) Boron
(D) Fluorine
The most electronegative element from the following elements is:
The tendency of an atom to attract electrons itself when combined in a covalent compound.
Study the extract of the Periodic Table given below and answer the questions that follow. Give the alphabet corresponding to the element in question. DO NOT repeat an element. 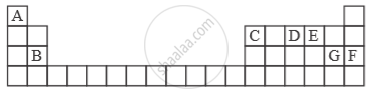
Which element forms electrovalent compound with G?
In terms of electronic configuration, what the elements of a given period and groups have in common?
Explain
K is more reactive than Li.
Answer the following question using the data given below:
- Assertion: The nature of bond in HF molecule is ionic
- Reason: The electronegativity difference between H and F is 1.9.
State the term for the following:
The tendency of an atom to pull a shared pair of electrons towards itself in a compound.
Electronegativity across the period ______.
A group of elements in the periodic table is given below (Boron is the first member of the group and Thallium is the last). Answer the following question in relation to the given group of elements:
Which element would be expected to have the highest electronegativity?
