Advertisements
Advertisements
प्रश्न
The threshold wavelength of tungsten is 2.76 x 10-5 cm.
(a) Explain why no photoelectrons are emitted when the wavelength is more than 2.76 x 10-5 cm.
(b) What will be the maximum kinetic energy of electrons ejected in each of the following cases
(i) if ultraviolet radiation of wavelength λ = 1.80 × 10-5 cm and
(ii) radiation of frequency 4 x 1015 Hz is made incident on the tungsten surface?
उत्तर
Data: λ0 = 2.76 x 10-5 cm = 2.76 x 10-7 m,
λ = 1.80 × 10-5 cm = 1.80 × 10-7 m,
v = 4 × 1015 Hz,
h = 6.63 × 10-34 J.s,
c = 3 × 108 m/s
(a) For λ > λ0,v < v0 (threshold frequency).
∴ hv < hv0. Hence, no photoelectrons are emitted.
(b) Maximum kinetic energy of electrons ejected
`= "hc" (1/lambda - 1/lambda_0)`
`= (6.63 xx 10^-34)(3 xx 10^8)(10^7/1.8 - 10^7/2.76)`J
= (6.63 × 10-19)(3)(0.5555 - 0.3623)
= (6.63)(3)(0.1932 × 10-19)J
= 3.842 × 10-19J
`= (3.842 xx 10^-19 "J")/(1.6 xx 10^-19 "J"//"eV")`
= 2.40 eV
(c) Maximum kinetic energy of electrons ejected
= hv - `"hc"/lambda_0`
`= (6.63 xx 10^-34)(4 xx 10^15) - ((6.63 xx 10^-34)(3 xx 10^8))/(2.76 xx 10^-7)`
= 26.52 × 10-19 - 7.207 × 10-19
= 19.313 × 10-19 J
= `(19.313 xx 10^19 "J")/(1.6 xx 10^-19 "J"//"eV")`
= 12.07 eV
संबंधित प्रश्न
Given the following data for incident wavelength and the stopping potential obtained from an experiment on the photoelectric effect, estimate the value of Planck's constant and the work function of the cathode material. What is the threshold frequency and corresponding wavelength? What is the most likely metal used for emitter?
| Incident wavelength (in Å) | 2536 | 3650 |
| Stopping potential (in V) |
1.95 | 0.5 |
The electrons are emitted in the photoelectric effect from a metal surface.
The maximum kinetic energy of the photoelectrons depends only on ______
Draw a neat labelled diagram of a schematic of the experimental setup for the photoelectric effect.
Explain the concept of the photoelectric effect.
With the help of a circuit diagram describe the experiment to study the characteristics of the photoelectric effect. Hence discuss any 2 characteristics of the photoelectric effect.
The maximum velocity of the photoelectron emitted by the metal surface is 'v '. Charge and mass of the photoelectron is denoted by 'e' and 'm' respectively. The stopping potential in volt is ______.
In photoelectric experiment, if both the intensity and frequency of the incident light are doubled, then the saturation of photoelectric current ______.
An important spectral emission line has a wavelength of 21 cm. The corresponding photon energy is (h = 6.62 x 10-34 Js, c = 3 x 108 m/s) ____________.
In photoelectric effect, for a light of different intensities but of same frequency, the stopping potential for a given metal is ____________.
Light of frequency 2 times the threshold frequency is incident on a photo sensitive material. If the frequency is made `1/3`rd and intensity is doubled then the photocurrent will ______.
When light of wavelength 'λ' is incident on a photosensitive surface, the stopping potential is 'V'. When light of wavelength '3λ' is incident on the same surface, the stopping potential is `"V"/6`. Threshold wavelength for the surface is _______.
The photo electric effect to take place for a metal, the minimum frequency required is 5.792 × 1014 Hz. A light of wavelength 6000 Å is incident on that metal surface. What is the corresponding frequency of light and will there be photoelectric emissions? [velocity of light = 3 × 108 m/s]
Photoelectrons are emitted from a photosensitive surface for the light of wavelengths λ1 = 360 nm and λ2 = 600 nm. What is the ratio of work functions for lights of wavelength 'λ1' to 'λ2'?
The photon of frequency vis incident on a metal surface whose threshold frequency is v0. The kinetic energy of the emitted photoelectrons will be ______.
The radiations of energies 1 eV and 2.5 eV are incident on a metal surface having work function 0.5 eV. The ratio of the maximum velocities of the emitted photo-electrons is ____________.
When light of wavelength '`lambda`' is incident on photosensitive surface, photons of power 'P' are emitted. The number of photons (n) emitted in 't' second is (h = Planck's constant, c = velocity of light in vacuum) ____________.
A metal surface having work function 'w0' emits photoelectrons when photons of energy 'E' are incident on it. The electron enters the uniform magnetic field (B) in perpendicular direction and moves in circular path of radius 'r'. Then 'r' is equal to (m and e be the mass and charge of electron respectively) ____________.
When the work function of a metal increases, maximum kinetic energy of emitted photoelectrons ____________.
Which one of the following graphs represents the variation of photoelectric current (i) with intensity (I) of the incident light?
The wavelength of light incident on a metal surface is reduced from 300 nm to 200 nm (both are less than threshold wavelength). What is the change in the stopping potential for photoelectrons emitted from the surface will be ______ V. (Take h = 6.6 × 10-34 J-s)
If the electron in hydrogen atom jumps from second Bohr orbit to ground state and difference between energies of the two states is radiated in the form of photons. If the work function of the material is 4.2 eV, then stopping potential is ______.
[Energy of electron in nth orbit = `-13.6/"n"^2` eV ]
On a photosensitive material when frequency of incident radiation is increased by 30%, kinetic energy of emitted photoelectrons increases from 0.4 eV. The work function of the surface is ______.
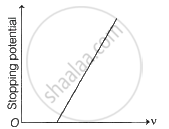
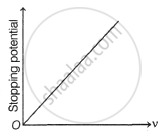
The following graphs show the variation of stopping potential corresponding to the frequency of incident radiation (ν) for a given metal. The correct variation is shown in graph [ν0 = threshold frequency].
|
(A) |
(B) |
|
(C) |
(D) |
The photoelectric threshold for a certain metal surface is 3600 Å. If the metal surface is irradiated by a wavelength of 1100 Å, then kinetic energy of the emitted photoelectrons is ______.
By increasing the voltage in an electron diffraction tube, the radius of the diffraction rings will ______.
The threshold frequency for a certain metal for photoelectric effect is 1.7 x 1015 Hz. When a light of frequency 2.2 x 1015 Hz is incident on the metal surface, the kinetic energy of the emitted photoelectrons is 3.3 x.10-19 J. Calculate Planck's constant.