Advertisements
Advertisements
प्रश्न
What does the diffusion of gases tell us about their particles?
उत्तर
The diffusion of gases tells us that:
- Matter consists of tiny particles, that have space between them.
- The particles of matter are in constant motion.
APPEARS IN
संबंधित प्रश्न
The mass per unit volume of a substance is called density.
(density = mass/volume).
Arrange the following in order of increasing density-
air, exhaust from chimneys, honey, water, chalk, cotton and iron.
Tabulate the differences in the characteristics of states of matter.
Give a reason for the following observation.
Naphthalene balls disappear with time without leaving any solid.
Write the full forms of the following
- LPG
- CNG
Why do gases diffuse very fast?
Why does a gas exert pressure?
When a gas jar containing colourless air is kept upside down over a gas jar full of brown-coloured bromine vapour, then after some time, the brown colour of bromine vapour spreads into the upper gas jar making both the gas jars appear brown in colour. Which of the following conclusion obtained from these observations is incorrect?
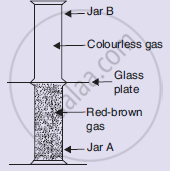
Look at the diagram on the right side. Jar A contains a red-brown gas whereas jar B contains a colourless gas. The two gas jars are separated by a galas plate placed between them
- What will happen when the glass plate between the two jars is pulled away?
- What name is given to the phenomenon which takes place?
- Name the brown gas which could be in jar A.
- Which is the colourless gas most likely to be present in jar B?
- Name one coloured solid and one colourless liquid which can show the same phenomenon.
Comment upon the following:-
rigidity
Comment upon the following:-
shape
