Advertisements
Advertisements
प्रश्न
Which is the real bleaching agent present in bleaching powder?
उत्तर
Chlorine is the real bleaching agent in bleaching powder. Calcium oxychloride, also known as bleaching powder (CaOCl2), is a yellowish white powder with a strong chlorine odour. Chlorine and calcium carbonate are the products of its reaction with atmospheric carbon dioxide.
APPEARS IN
संबंधित प्रश्न
What is the common name of the compound CaOCl2?
Name the substance obtained by the action of chlorine on solid (dry) slaked lime.
What is the common name of the compound CaOCl2?
What is bleaching powder?
What happens when bleaching powder reacts with dilute sulphuric acid? Give equation of the reaction involved.
State two important uses of bleaching powder.
When chlorine and sodium hydroxide being produced during the electrolysis of brine are allowed to mix, a new chemical is formed. Name the chemical and write its uses.
Brine is an ____________.
CaOCl2 will liberate Cl2 gas in the presence of
(i) CO2
(ii) HCl
(iii) CO
(iv) NO
The reaction between MnO2 with HCl is depicted in the following diagram. It was observed that gas with bleaching abilities was released.
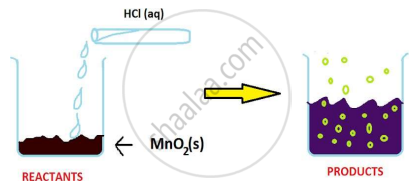
The chemical reaction between MnO2 and HCl is an example of:
