Advertisements
Advertisements
प्रश्न
Which of the following graphs is correct for a first order reaction?

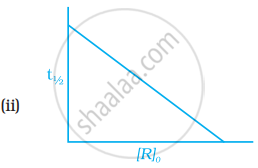
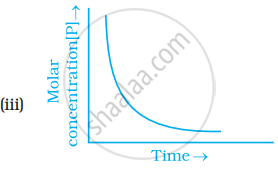
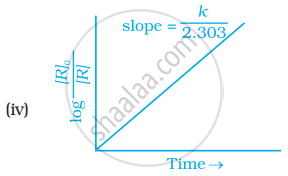
उत्तर


Explanation:
The pace of a reaction that is directly proportional to the concentration of the reacting substance is known as a first-order reaction.
A first-order reaction's kinetic equation
k = `2.303/t log [R]^0/([R])`
The Y-axis represents concentration, whereas the X-axis represents time.
`log = [R]^0/([R]) = (kt)/2.303`
`[log = [R]_0/([R])] = [(kt)/2.303](t)`
y = mn
k = `2.303/t log a/(a - x)`
t = `2.303/k log a/(a - x)`
x = `a - a/2`
x = `a/2`
`t_(1/2) = 2.303/k log a/(a - a/2)`
`2.303/k log 2`
`t_(1/2) = 0.693/k`
APPEARS IN
संबंधित प्रश्न
The rate constant for a first order reaction is 60 s−1. How much time will it take to reduce the initial concentration of the reactant to its `1/16`th value?
Show that the time required for 99.9% completion of a first-order reaction is three times the time required for 90% completion.
Straight line graph for first order reaction is obtained between ____________.
In the presence of acid, the initial concentration of cane sugar was reduced from 0.2 M to 0.1 Min 5 hours and to 0.05 Min 10 hours. The reaction must be of?
First order reaction is 50% complete in 1.26 × 1014s. How much time could it take for 100% completion?
A first order reaction is 50% complete in 20 minute What is rate constant?
The decomposition of formic acid on gold surface follows first-order kinetics. If the rate constant at 300 K is 1.0 × 10−3 s−1 and the activation energy Ea = 11.488 kJ mol−1, the rate constant at 200 K is ______ × 10−5 s−1. (Round off to the Nearest Integer)
(Given R = 8.314 J mol−1 K−1)
For a first order reaction, the ratio of the time for 75% completion of a reaction to the time for 50% completion is ______. (Integer answer)
How will you represent first order reactions graphically?
What is the rate constant?
