Advertisements
Advertisements
प्रश्न
Which of the two structures (A) and (B) given below is more stabilised by resonance? Explain.
\[\ce{\underset{(A)}{CH3COOH}}\] and \[\ce{\underset{(B)}{CH3CO\overset{Θ}{O}}}\]
उत्तर
Resonating structures are as follows:
| (A) |  |
| (B) | 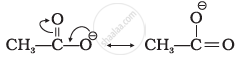 |
Structure 'B' is more stabilised as it does not involve charge separation.
APPEARS IN
संबंधित प्रश्न
Draw the resonance structure for the following compound. Show the electron shift using curved-arrow notation.
C6H5NO2
Draw the resonance structure for the following compound. Show the electron shift using curved-arrow notation.
CH3CH = CHCHO
Draw the resonance structure for the following compound. Show the electron shift using curved-arrow notation.
\[\ce{CH3CH = CH\overset{+}{C}H2}\]
In which of the following representations given below spatial arrangement of group/ atom different from that given in structure ‘A’?
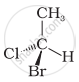 |
| (A) |
| (i) | 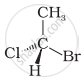 |
| (ii) | 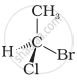 |
| (iii) | 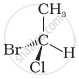 |
| (iv) | 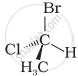 |
Show the polarisation of carbon-magnesium bond in the following structure.
CH3 – CH2 – CH2 – CH2 – Mg – X
Draw the possible resonance structures for \[\ce{CH3 - \overset{\bullet\bullet}{\underset{\bullet\bullet}{O}} - \overset{+}{C}H2}\] and predict which of the structures is more stable. Give reason for your answer.
Draw the resonance structure of the following compounds;
CH2 = CH – CH = CH2
Draw the resonance structure of the following compounds;
\[\begin{array}{cc}
\ce{CH2 = CH - C = O}\\
\phantom{.........}|\\
\phantom{.........}\ce{H}
\end{array}\]
Resonance structures of propenal are given below. Which of these resonating structures is more stable? Give reason for your answer.
\[\ce{\underset{I}{CH2 = CH - CH = O} <-> \underset{II}{\overset{⊕}{C}H2 - CH = CH - \overset{Θ}{O}}}\]
Assertion (A): Energy of resonance hybrid is equal to the average of energies of all canonical forms.
Reason (R): Resonance hybrid cannot be presented by a single structure.
